Abstract
Objectives
The amount and distribution of intratumoural collagen fibre vary among different thymic tumours, which can be clearly detected with T2- and diffusion-weighted MR images. To explore the incidences of collagen fibre patterns (CFPs) among thymomas, thymic carcinomas and lymphomas on imaging, and to evaluate the efficacy and reproducibility of CFPs in differential diagnosis of thymic tumours.
Materials and methods
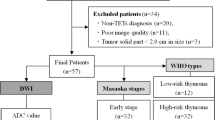
Three hundred and ninety-eight patients with pathologically diagnosed thymoma, thymic carcinoma and lymphoma who underwent T2- and diffusion-weighted MR imaging were retrospectively enrolled. CFPs were classified into four categories: septum sign, patchy pattern, mixed pattern and no septum sign. The incidences of CFPs were compared among different thymic tumours, and the efficacy and reproducibility in differentiating the defined tumour types were analysed.
Results
There were significant differences in CFPs among thymomas, thymic squamous cell carcinomas (TSCCs), other thymic carcinomas and neuroendocrine tumours (OTC&NTs) and thymic lymphomas. Septum signs were found in 209 (86%) thymomas, which differed between thymomas and any other thymic neoplasms (all p < 0.005). The patchy, mixed patterns and no septum sign were mainly seen in TSCCs (80.3%), OTC&NTs (78.9%) and thymic lymphomas (56.9%), respectively. The consistency of different CFP evaluation between two readers was either good or excellent. CFPs achieved high efficacy in identifying the thymic tumours.
Conclusion
The CFPs based on T2- and diffusion-weighted MR imaging were of great value in the differential diagnosis of thymic tumours.
Key Points
• Significant differences are found in intratumoural collagen fibre patterns among thymomas, thymic squamous cell carcinomas, other thymic carcinomas and neuroendocrine tumours and thymic lymphomas.
• The septum sign, patchy pattern, mixed pattern and no septum sign are mainly seen in thymomas (86%), thymic squamous cell carcinomas (80.3%), other thymic carcinomas and neuroendocrine tumours (79%) and thymic lymphomas (57%), respectively.
• The collagen fibre patterns have high efficacy and reproducibility in differentiating thymomas, thymic squamous cell carcinomas and thymic lymphomas.






Similar content being viewed by others
Abbreviations
- CFP:
-
Collagen fibre pattern
- DWI:
-
Diffusion-weighted imaging
- FS-T2WI:
-
Fat-suppressed T2 weighted imaging
- GCTs:
-
Germ cell tumours
- NPV:
-
Negative predictive value
- OTC&NT:
-
Other thymic carcinoma and neuroendocrine tumour
- PPV:
-
Positive predictive value
- TSCC:
-
Thymic squamous cell carcinoma
- WHO:
-
World Health Organization
References
Marx A, Chan JK, Coindre JM et al (2015) The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol 10:1383–1395
Ried M, Marx A, Gotz A, Hamer O, Schalke B, Hofmann HS (2016) State of the art: diagnostic tools and innovative therapies for treatment of advanced thymoma and thymic carcinoma. Eur J Cardiothorac Surg 49:1545–1552
Lee HS, Jang HJ, Shah R et al (2017) Genomic analysis of thymic epithelial tumors identifies novel subtypes associated with distinct clinical features. Clin Cancer Res 23:4855–4864
Yano M, Sasaki H, Yukiue H et al (2009) Thymoma with dissemination: efficacy of macroscopic total resection of disseminated nodules. World J Surg 33:1425–1431
Hakiri S, Kawaguchi K, Fukui T et al (2019) Verification of the diagnostic strategy for anterior mediastinal tumors. Int J Clin Oncol 24:385–393
Kang CH, Kim YT, Jheon SH, Sung SW, Kim JH (2008) Surgical treatment of malignant mediastinal nonseminomatous germ cell tumor. Ann Thorac Surg 85:379–384
Ozawa Y, Hara M, Shimohira M, Sakurai K, Nakagawa M, Shibamoto Y (2016) Associations between computed tomography features of thymomas and their pathological classification. Acta Radiol 57:1318–1325
Sadohara J, Fujimoto K, Muller NL et al (2006) Thymic epithelial tumors: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol 60:70–79
Jing Y, Yan WQ, Li GF et al (2018) Usefulness of volume perfusion computed tomography in differentiating histologic subtypes of thymic epithelial tumors. J Comput Assist Tomogr 42:594–600
Azizad S, Sannananja B, Restrepo CS (2016) Solid tumors of the mediastinum in adults. Semin Ultrasound CT MR 37:196–211
Benveniste MF, Rosado-de-Christenson ML, Sabloff BS, Moran CA, Swisher SG, Marom EM (2011) Role of imaging in the diagnosis, staging, and treatment of thymoma. Radiographics 31:1847–1861 discussion 1861-1843
Yabuuchi H, Matsuo Y, Abe K et al (2015) Anterior mediastinal solid tumours in adults: characterisation using dynamic contrast-enhanced MRI, diffusion-weighted MRI, and FDG-PET/CT. Clin Radiol 70:1289–1298
Carter BW, Benveniste MF, Truong MT, Marom EM (2015) State of the art: MR imaging of thymoma. Magn Reson Imaging Clin N Am 23:165–177
Carter BW, Okumura M, Detterbeck FC, Marom EM (2014) Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol 9:S110–S118
Gumustas S, Inan N, Sarisoy HT et al (2011) Malignant versus benign mediastinal lesions: quantitative assessment with diffusion weighted MR imaging. Eur Radiol 21:2255–2260
Takahashi K, Al-Janabi NJ (2010) Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging 32:1325–1339
Tomiyama N, Honda O, Tsubamoto M et al (2009) Anterior mediastinal tumors: diagnostic accuracy of CT and MRI. Eur J Radiol 69:280–288
Erasmus JJ, McAdams HP, Donnelly LF, Spritzer CE (2000) MR imaging of mediastinal masses. Magn Reson Imaging Clin N Am 8:59–89
Li B, Xin YK, Xiao G et al (2019) Predicting pathological subtypes and stages of thymic epithelial tumors using DWI: value of combining ADC and texture parameters. Eur Radiol 29:5330–5340
Abdel Razek AA, Khairy M, Nada N (2014) Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology 273:268–275
Li GF, Duan SJ, Yan LF et al (2017) Intravoxel incoherent motion diffusion-weighted MR imaging parameters predict pathological classification in thymic epithelial tumors. Oncotarget 8:44579–44592
Priola AM, Priola SM, Giraudo MT et al (2015) Diffusion-weighted magnetic resonance imaging of thymoma: ability of the apparent diffusion coefficient in predicting the World Health Organization (WHO) classification and the Masaoka-Koga staging system and its prognostic significance on disease-free survival. Eur Radiol 26:2126–2138
Xiao G, Hu YC, Ren JL et al (2020) MR imaging of thymomas: a combined radiomics nomogram to predict histologic subtypes. Eur Radiol. https://doi.org/10.1007/s00330-020-07074-3
Sakai F, Sone S, Kiyono K et al (1992) MR imaging of thymoma: radiologic-pathologic correlation. AJR Am J Roentgenol 158:751–756
Wang M, Kundu U, Gong Y (2020) Pitfalls of FNA diagnosis of thymic tumors. Cancer Cytopathol 128:57–67
Hu YC, Wu L, Yan LF et al (2014) Predicting subtypes of thymic epithelial tumors using CT: new perspective based on a comprehensive analysis of 216 patients. Sci Rep 4:6984
Moran CA, Weissferdt A, Kalhor N et al (2012) Thymomas I: a clinicopathologic correlation of 250 cases with emphasis on the World Health Organization schema. Am J Clin Pathol 137:444–450
Travis WDBE, Müller-Hermelink HK, Harris CC (2004) World Health Organization classification of tumours. Pathology and genetics of tumours of the lung, thymus and heart. IARC Press, Lyon, pp 152–153
Jeong YJ, Lee KS, Kim J, Shim YM, Han J, Kwon OJ (2004) Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol 183:283–289
Inoue A, Tomiyama N, Fujimoto K et al (2006) MR imaging of thymic epithelial tumors: correlation with World Health Organization classification. Radiat Med 24:171–181
Huang J, Ahmad U, Antonicelli A et al (2014) Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol 9:1573–1578
Yan WQ, Xin YK, Jing Y et al (2018) Iodine quantification using dual-energy computed tomography for differentiating thymic tumors. J Comput Assist Tomogr 42:873–880
Xiao G, Rong WC, Hu YC et al (2019) MRI radiomics analysis for predicting the pathologic classification and TNM staging of thymic epithelial tumors: a pilot study. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.19.21696:1-13
Kushihashi T, Fujisawa H, Munechika H (1996) Magnetic resonance imaging of thymic epithelial tumors. Crit Rev Diagn Imaging 37:191–259
Shimamoto A, Ashizawa K, Kido Y et al (2017) CT and MRI findings of thymic carcinoid. Br J Radiol 90:20150341
Lausi PO, Refai M, Filosso PL et al (2014) Thymic neuroendocrine tumors. Thorac Surg Clin 24:327–332
Funding
This study has received funding by the Science and Technology Innovation Development Foundation of Tangdu Hospital (No. 2017LCYJ004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Guang-bin Cui.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in 77, 57, 189 and 182 thymic epithelial tumour patients, respectively [1–4]. Four previous articles explored the values of intravoxel incoherent motion DWI [1], DWI texture parameters [2] and MRI radiomics [3], and combined radiomics nomogram [4] in predicting the pathological classification of thymic epithelial tumours, whereas in this manuscript we reported the usefulness of CFPs in differentiating thymic tumours.
1. Li GF, Duan SJ, Yan LF et al (2017) Intravoxel incoherent motion diffusion-weighted MR imaging parameters predict pathological classification in thymic epithelial tumours. Oncotarget 8:44579-44592
2. Li B, Xin YK, Xiao G et al (2019) Predicting pathological subtypes and stages of thymic epithelial tumours using DWI: value of combining ADC and texture parameters. Eur Radiol 29:5330-5340
3. Xiao G, Rong WC, Hu YC et al (2019) MRI Radiomics Analysis for Predicting the Pathologic Classification and TNM Staging of Thymic Epithelial Tumors: A Pilot Study. AJR Am J Roentgenol. 10.2214/AJR.19.21696:1-13
4. Xiao G, Hu YC, Ren JL et al (2020) MR imaging of thymomas: a combined radiomics nomogram to predict histologic subtypes. Eur Radiol. 10.1007/s00330-020-07074-3.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, YC., Yan, WQ., Yan, LF. et al. Differentiating thymoma, thymic carcinoma and lymphoma based on collagen fibre patterns with T2- and diffusion-weighted magnetic resonance imaging. Eur Radiol 32, 194–204 (2022). https://doi.org/10.1007/s00330-021-08143-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08143-x