Abstract
Objectives
To determine the incidence, risk factors, and prognostic indicators of symptomatic air embolism after percutaneous transthoracic lung biopsy (PTLB) by conducting a systematic review and pooled analysis.
Methods
We searched the EMBASE and OVID-MEDLINE databases to identify studies that dealt with air embolism after PTLB and had extractable outcomes. The incidence of air embolism was pooled using a random effects model, and the causes of heterogeneity were investigated. To analyze risk factors for symptomatic embolism and unfavorable outcomes, multivariate logistic regression analysis was performed.
Results
The pooled incidence of symptomatic air embolism after PTLB was 0.08% (95% confidence interval [CI], 0.048–0.128%; I2 = 45%). In the subgroup analysis and meta-regression, guidance modality and study size were found to explain the heterogeneity. Of the patients with symptomatic air embolism, 32.7% had unfavorable outcomes. The presence of an underlying disease (odds ratio [OR], 5.939; 95% CI, 1.029–34.279; p = 0.046), the use of a ≥ 19-gauge needle (OR, 10.046; 95% CI, 1.103–91.469; p = 0.041), and coronary or intracranial air embolism (OR, 19.871; 95% CI, 2.725–14.925; p = 0.003) were independent risk factors for symptomatic embolism. Unfavorable outcomes were independently associated with the use of aspiration biopsy rather than core biopsy (OR, 3.302; 95% CI, 1.149–9.492; p = 0.027) and location of the air embolism in the coronary arteries or intracranial spaces (OR = 5.173; 95% CI = 1.309–20.447; p = 0.019).
Conclusion
The pooled incidence of symptomatic air embolism after PTLB was 0.08%, and one-third of cases had sequelae or died. Identifying whether coronary or intracranial emboli exist is crucial in suspected cases of air embolism after PTLB.
Key Points
• The pooled incidence of symptomatic air embolism after percutaneous transthoracic lung biopsy was 0.08%, and one-third of patients with symptomatic air embolism had sequelae or died.
• The risk factors for symptomatic air embolism were the presence of an underlying disease, the use of a ≥ 19-gauge needle, and coronary or intracranial air embolism.
• Sequelae and death in patients with symptomatic air embolism were associated with the use of aspiration biopsy and coronary or intracranial locations of the air embolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous transthoracic lung biopsy (PTLB) is a well-established image-guided procedure for the diagnosis of lung parenchymal lesions [1, 2] with excellent diagnostic accuracy [3, 4]. Nevertheless, PTLB can be accompanied by complications, including pneumothorax (9 to 54% with an average of around 20%), lung parenchymal hemorrhage (11%), and hemoptysis (5.2 to 7%) [5,6,7]. The majority of these complications can be managed conservatively or with a minimal intervention, such as percutaneous tube drainage, without any sequelae [5, 6, 8].
Air embolism is another complication of PTLB [6,7,8,9,10,11]. It occurs extremely rarely [12,13,14], but can result in mortality or sequelae [15,16,17], and therefore constitutes a significant concern for radiologists. However, recent studies have reported cases with symptomatic air embolism after PTLB that resolved without sequelae [6, 8, 18, 19]. Due to the rarity of air embolism, most of the relevant publications reported a single case or a few cases, hampering a comprehensive understanding of air embolism after PTLB. A systematic review, followed by a pooled analysis, is a way to synthesize information from all the relevant publications regarding air embolism after PTLB. Thus, we conducted a systematic review and pooled analysis to determine the incidence, risk factors, and prognostic indicators of symptomatic air embolism after PTLB.
Materials and methods
This systematic review was performed and reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol of this study was registered in the International Prospective Register of Systematic Reviews (ID: CRD42019141479).
Search strategy
We searched the EMBASE and OVID-MEDLINE databases to identify relevant publications with the following search terms: [(lung OR pulmonary) AND {(percutaneous OR transthora*) OR (biops* OR aspira* OR needle)}] AND {(air AND embol*) OR (gas AND embol*)}. The initial search was undertaken on May 8, 2019, and updated on January 5, 2020. We only included articles published in English without any limits on publication year.
Eligible criteria for study selection
We reviewed all the searched publications regardless of the study type. The inclusion criteria were as follows: (a) studies with one or more patients suspected of having air embolism after PTLB, (b) confirmation of the presence of air embolism by autopsy or computed tomography (CT), and (c) sufficient data described in detail to extract the final outcomes and the variables associated with air embolism. We applied the following exclusion criteria: (a) studies with individuals who underwent bronchoscopic lung biopsy, not PTLB, and (b) individuals who underwent percutaneous needle localization, rather than biopsy.
We only included case reports, case series, prospective or retrospective cohort studies, and case-control studies, not letters, editorial comments, abstracts, review articles, guidelines, or consensus statements. Full-text articles were assessed for eligibility independently by two authors (J.H.L. and S.H.Y. with 7 and 14 years of experience in thoracic radiology, respectively). Any discrepancy was resolved by consensus.
Data extraction
For data extraction, the two authors (J.H.L. and S.H.Y.) independently reviewed all eligible articles and extracted the following data using a standardized spreadsheet in Microsoft Excel 2016 (Microsoft Corporation): study characteristics, population characteristics, radiological characteristics of the biopsied lesion, information related to PTLB, information related to air embolism, and prognosis. In addition to this review, we tried to contact the corresponding authors of each study via e-mail for missing or unreported data.
Methodological quality assessment of individual studies
Quality assessment was performed according to the tool proposed by Murad et al, which is composed of eight items categorized into four domains [20]. Three items in the causality domain were not adopted because they are mostly relevant to cases of adverse drug events [20]. We assessed the methodological quality of individual studies based on the five remaining items according to each purpose, which dealt with selection, ascertainment of exposure, causality, and reporting [20].
Data synthesis and statistical analysis
We estimated the pooled incidence using a random-intercept logistic regression model, which is preferred over conventional approximate methods, especially for rare events [21]. Heterogeneity across the included studies was evaluated using the I2 statistic, which was derived from the Cochran Q statistic using the following equation, I2 = 100% · (Q−df)/Q. An I2 statistic > 50% was regarded as indicating substantial heterogeneity. A subgroup analysis and meta-regression were performed to investigate the causes of heterogeneity with the following covariates: guidance modality (CT guidance versus other modalities), study size (≥ 2500 vs. < 2500), and publication year (before 2000 vs. after 2000). The potential for publication bias was evaluated through a visual assessment of funnel plots of sample size against incidence due to the sparse event data [22].
We extracted individual-level data on patients with symptomatic or asymptomatic embolism from the included publications and analyzed the risk factors and prognostic indicators of symptomatic air embolism after PTLB. Unfavorable outcomes were defined as (a) patients’ survival with sequelae or (b) death. Since it was obvious that asymptomatic patients with air embolism after PTLB had favorable outcomes, we only included symptomatic patients in the analysis of unfavorable outcomes. The symptoms and locations of air embolism were categorized according to whether the brain or heart was involved because these organs play a critical role in patients’ outcomes [15,16,17]. For the univariate analysis, the Student t test for continuous variables and the Pearson chi-square test or Fisher exact test for categorical variables were performed. Subsequently, multivariate logistic regression analysis with backward stepwise selection was performed to identify predictors of symptomatic air embolism and unfavorable outcomes, respectively, using variables with a p value < 0.1 in the univariate analysis. Backward stepwise selection was conducted with an iterative entry of variables based on the test results (p < 0.05), and the removal of variables was based on likelihood ratio statistics with a probability of 0.10.
A p value of < 0.05 was considered to indicate a statistically significant difference, and statistical analyses were performed using SPSS version 21.0 (IBM Corp.) and R version 3.6.1 (R Foundation for Statistical Computing).
Results
Eligible studies and methodological quality
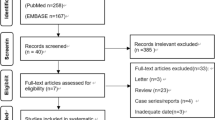
The initial literature search identified 1756 studies, of which 94 were eligible for the analysis of the incidence of symptomatic air embolism after PTLB or risk factors for symptomatic embolism and unfavorable outcomes. A supplemental search of the bibliographies of the retrieved publications added 7 studies. Finally, 101 studies were included in our analysis (Fig. 1).
In the methodological quality assessment, 19 articles with information on the incidence of symptomatic air embolism [3, 5, 6, 9, 10, 14, 18, 19, 23,24,25,26,27,28,29,30,31,32,33] and 90 articles with information on the risk factors for symptomatic air embolism and unfavorable outcomes were evaluated [6, 9, 14,15,16,17,18,19, 24, 26, 29, 34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112], respectively. For the incidence of symptomatic air embolism, all 19 articles were considered high quality, meeting all four items adequately. In the evaluation of risk factors, 81 of 90 articles were judged as high quality, as they satisfied all four items. However, seven case reports did not establish a causality between air embolism and PTLB because the air embolism occurred after repositioning of the biopsy needle to target the lung lesions before firing the biopsy needle [16, 24, 39, 53, 61, 89, 106]. Another case report was judged to have insufficient establishment of causality because it described a patient with air embolism just after removal of the coaxial needle stylet [75]. The last case report lacked an adequate ascertainment of exposure and causality between air embolism and PTLB because the patient only had local anesthesia with lidocaine and then presented with symptoms [63].
Incidence of symptomatic air embolism of PTLB
Nineteen articles were included in the analysis of the incidence of symptomatic air embolism after PTLB, and the pooled incidence was 0.08% (95% confidence interval [CI], 0.048–0.128; I2 = 45%) (Fig. 2). The subgroup analysis and meta-regression (Table 1) showed that CT guidance (vs. other modalities; p = 0.002) and study size < 2500 (vs. ≥ 2500; p < 0.001) were significant factors explaining heterogeneity across studies. There was no evidence of publication bias on a visual evaluation of funnel plots (Fig. 3).
Characteristics of individual patient data
Details on the clinical, radiological, and biopsy-related information that could be matched to each individual from the 123 patients described in the 90 eligible articles are presented in Table 2.
Seventy-two of the 123 patients (58.5%) had underlying diseases, of which cancer was the most common (36.1%, 26 of 72), followed by chronic obstructive pulmonary disease (COPD) (23.6%, 17 of 72). Other diseases were asthma, idiopathic pulmonary fibrosis, hypertension, diabetes, and pneumoconiosis.
For 20 patients with lateral decubitus position during the procedure, targeted lungs were non-dependent in 18 patients while 2 patients had unspecified information of whether targeted lung was non-dependent or dependent.
Ninety-eight of the 123 patients (79.7%) had immediate symptoms or signs during or after PTLB. The most common symptom was loss of consciousness (42.9%, 42 of 98), followed by cardiac-related symptoms or signs (32.7%, 32 of 98; chest pain and myocardial infarction) and neurological symptoms or signs (24.5%, 24 of 98; e.g., reduction of sensation, motor weakness, dysarthria, blindness, dysarthria, convulsion). Other symptoms or signs included a change in blood pressure, voiding problems, dyspnea, and hemoptysis.
In terms of the location of the air embolism, 69 patients had air emboli in the coronary arteries or intracranial spaces (59%, 69 of 117). Other reported locations included the aorta (21.4%, 25 of 117), left atrium (17.1%, 20 of 117), left ventricle (19.7%, 23 of 117), right atrium (1.7%, 2 of 117), right ventricle (0.9%, 1 of 117), pulmonary artery (1.7%, 2 of 117), pulmonary vein (5.1%, 6 of 117), mesenteric artery (0.9%, 1 of 117), spinal artery (0.9%, 1 of 117), and the systematic venous system including the superior vena cava and subclavian vein (0.9%, 1 of 117).
In regard to patients’ outcomes, 66 of the 98 patients (67.3%) with symptomatic embolism survived without any sequelae, whereas 11 (11.2%) and 21 (21.5%) patients survived with sequelae and died, respectively. Therefore, 32 of 98 patients (32.7%) with symptomatic embolism were considered to have an unfavorable outcome.
Risk factors for symptomatic air embolism after PTLB
In the analysis of risk factors for symptomatic air embolism after PTLB, 117 patients were included after the exclusion of 6 patients whose symptoms or signs were not described in the corresponding studies. In the univariate analysis, a younger age than 65 years (p = 0.014), aspiration biopsy rather than core biopsy (p = 0.04), a ≥ 19-gauge needle (p = 0.004), and location of the air embolism in the coronary arteries or intracranial space (p < 0.001) showed significant associations with symptomatic air embolism while factors with p values between 0.05 and 0.1 were the presence of an underlying disease (p = 0.091) and supine position rather than lateral decubitus position (p = 0.094) (Table 3). In a multivariate analysis of 93 patients who had clear information on the above characteristics, the presence of an underlying disease (odds ratio [OR] = 5.939; 95% confidence interval [CI] = 1.029–34.279; p = 0.046), use of a ≥ 19-gauge needle (OR = 10.046, 95% CI = 1.103–91.469; p = 0.041), and location of the air embolism in the coronary arteries or intracranial spaces (OR = 19.871; 95% CI = 2.725–14.925; p = 0.003) were found to be significant risk factors. Regarding the underlying disease, cancer, COPD, hypertension, diabetes, idiopathic pulmonary fibrosis, and asthma were associated with symptomatic air embolism in descending order.
Prognostic indicators for unfavorable outcomes of air embolism after PTLB
In the analysis of unfavorable outcomes in patients with symptomatic air embolism, aspiration biopsy rather than core biopsy (p = 0.013), loss of consciousness as an immediate symptom (p = 0.021), and location of the air embolism in the coronary arteries or intracranial spaces (p = 0.001) were found to be significant risk factors in the univariate analysis, while factors with p values between 0.05 and 0.1 were a younger age than 65 years (p = 0.075), lesion size of 2 cm or larger (p = 0.093), no use of the coaxial technique (p = 0.087), and no application of the Trendelenburg position (p = 0.084) (Table 4). Excluding the factors of lesion size and use of the coaxial technique because of the large number of patients with missing data on these factors, a multivariate analysis could be performed of 90 patients. In this analysis, aspiration biopsy rather than core biopsy (OR = 3.302; 95% CI = 1.149–9.492; p = 0.027) and location of the air embolism in the coronary arteries or intracranial spaces (OR = 5.173; 95% CI = 1.309–20.447; p = 0.019) were significant risk factors for unfavorable outcomes.
Discussion
The commonly accepted incidence of air embolism, focusing on symptomatic patients, has been reported to be 0.02 to 0.07% [12,13,14], and our finding of a pooled incidence of symptomatic air embolism after PTLB of 0.08% is similar to the incidence reported in previous studies. However, some recent studies have reported that the incidence of air embolism after PTLB ranged from 0.21 to 3.8% based on the inclusion of asymptomatic patients with extensive surveys of post-biopsy CT examinations [6, 8, 9, 11, 19, 24]. Because of the major difference between the incidence of asymptomatic and symptomatic embolism unveiled in prior studies, we only pooled studies dealing with symptomatic air embolism.
In the subgroup analysis and meta-regression analysis, studies that only analyzed CT-guided PTLB had significantly more events than studies using other modalities (e.g., fluoroscopy and ultrasonography). The finding is unsurprising because air embolism could be detected directly with post-biopsy CT surveillance in studies of CT-guided PTLB. To support this inference, the majority of cases in those studies included cases of air embolism located in the heart, coronary arteries, aorta, and pulmonary vein, which are in the scan range of chest CT. Study size was another significant factor contributed to the heterogeneity, as the average incidence in studies that enrolled a small number of patients was higher than that of large-scale studies. It is speculated that when air embolism occurs after PTLB in a small-scale study, its incidence may be estimated as much higher than would be the case in a large-scale study.
Since prompt recognition of air embolism is critical for optimizing patients’ clinical course [6, 18], it is paramount to investigate the risk factors of symptomatic air embolism. We found the presence of an underlying disease, larger gauge of the biopsy needle, and location of the air embolism in the coronary arteries or intracranial spaces were predictive factors for symptomatic air embolism. COPD was the most common underlying disease (23.6% of the included patients), which may be explained by the fact that COPD can result in extended exposure of the pulmonary vessel lumen to the airway, thereby increasing the chance of air bubbles entering the bloodstream [56]. The relationship of needle gauge with air embolism after PTLB is controversial, because a number of cases of air embolism occurred even when a small needle was used [8, 72, 77, 78]. To explain the association found in our study, it is speculated that once an air embolism occurs, the use of a large needle results in higher chance of a sufficiently large air bolus entering the bloodstream and causing symptoms [9]. Finally, the presence of an air embolism in the coronary or cerebral arteries was linked to a severely deteriorated prognosis, with outcomes including myocardial infarction and permanent neurological deficits [6, 61, 85, 111]. Concordant with prior studies, location of the air embolism in the coronary arteries or intracranial spaces was a significant predictor of symptomatic air embolism.
Of note, we found that 32.7% (32 of 98) of symptomatic patients with air embolism had unfavorable outcomes. Of those 32 patients, 21 died and 11 survived, but had neurological sequelae. Our results suggest that two-thirds of patients with symptoms or signs eventually survive without any sequelae. The use of aspiration biopsy rather than core biopsy and the presence of the air embolism in coronary arteries or intracranial spaces were significant prognostic indicators for unfavorable outcomes in our study. We suggest that aspiration biopsy may cause a large air bolus due to the back-and-forth technique inherently involved in this procedure, which results in a high likelihood of air entering the bloodstream. As was described above, the organ involved in air embolism is a critical factor for patients’ symptoms and outcomes, and it is therefore natural for location of the air embolism in the coronary arteries and intracranial spaces to be a significant predictor of unfavorable outcomes.
There has been controversy about application of the Trendelenburg position for patients with air embolism [61, 90, 111, 113,114,115]. According to some authorities, the Trendelenburg position is only appropriate for cases of venous air embolism, as it keeps the air bolus in the right ventricular cavity [114, 115]. Nevertheless, our systematic review revealed that patients who had been placed in the Trendelenburg position for air embolism tended to have favorable outcomes (20 of 25 patients, p = 0.084 in the univariate analysis) and most of these patients had arterial air embolism (23 of 25 patients). Although application of the Trendelenburg position did not show significance in the multivariate analysis, this tendency of the Trendelenburg position to be associated with favorable outcomes should be investigated in further studies.
Two main limitations should be stated. Most importantly, this study comprised mostly case reports or series due to the rarity of air embolism after PTLB, and therefore, the certainty of the evidence could be low [20]. For instance, hyperbaric oxygen therapy is believed to be a mainstay treatment for air embolism because it helps reduce bubble volume, biochemical reactions at the blood-gas interface that result in hemostasis, and endothelial damage [69, 79, 90]. Despite its proven usefulness, it was not associated with patients’ outcomes in our study. Second, we did not receive clarifications on missing or unreported data in several cases. Consequentially, relatively few patients were included in the analysis of risk factors, which might have caused potential bias and limited our results.
In conclusion, the pooled incidence of symptomatic air embolism after PTLB was 0.08%, and one-third of cases had sequelae or died. Symptomatic air embolism was more frequent in patients with an underlying disease (p = 0.046), with the use of a ≥ 19-gauge needle (p = 0.041), and when the air embolism was located in the coronary arteries or intracranial spaces (p = 0.003). The use of aspiration biopsy rather than core biopsy (p = 0.027) and location of the air embolism in the coronary arteries or intracranial spaces (p = 0.019) were independent prognostic indicators of unfavorable outcomes.
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- PTLB:
-
Percutaneous transthoracic lung biopsy
References
Rivera MP, Mehta AC, Wahidi MM (2013) Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e142S–e165S
Manhire A, Charig M, Clelland C et al (2003) Guidelines for radiologically guided lung biopsy. Thorax 58:920–936
Lee SM, Park CM, Lee KH, Bahn YE, Kim JI, Goo JM (2013) C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology 271:291–300
Hiraki T, Mimura H, Gobara H et al (2009) CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest 136:1612–1617
Yoon SH, Park CM, Lee KH et al (2019) Analysis of complications of percutaneous transthoracic needle biopsy using CT-guidance modalities in a multicenter cohort of 10568 biopsies. Korean J Radiol 20:323–331
Jang H, Rho JY, Suh YJ, Jeong YJ (2019) Asymptomatic systemic air embolism after CT-guided percutaneous transthoracic needle biopsy. Clin Imaging 53:49–57
Boskovic T, Stanic J, Pena-Karan S et al (2014) Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 6:S99–S107
Hiraki T, Fujiwara H, Sakurai J et al (2007) Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest 132:684–690
Ishii H, Hiraki T, Gobara H et al (2014) Risk factors for systemic air embolism as a complication of percutaneous CT-guided lung biopsy: multicenter case-control study. Cardiovasc Intervent Radiol 37:1312–1320
Glodny B, Schonherr E, Freund MC et al (2017) Measures to prevent air embolism in transthoracic biopsy of the lung. AJR Am J Roentgenol 208:W184–W191
Freund MC, Petersen J, Goder KC, Bunse T, Wiedermann F, Glodny B (2012) Systemic air embolism during percutaneous core needle biopsy of the lung: frequency and risk factors. BMC Pulm Med. https://doi.org/10.1186/1471-2466-12-2
Sinner WN (1976) Complications of percutaneous transthoracic needle aspiration biopsy. Acta Radiol Diagn (Stockh) 17:813–828
Richardson CM, Pointon KS, Manhire AR, Macfarlane JT (2002) Percutaneous lung biopsies: a survey of UK practice based on 5444 biopsies. Br J Radiol 75:731–735
Tomiyama N, Yasuhara Y, Nakajima Y et al (2006) CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 59:60–64
Cheng HM, Chiang KH, Chang PY et al (2010) Coronary artery air embolism: a potentially fatal complication of CT-guided percutaneous lung biopsy. Br J Radiol 83:e83–e85
Tolly TL, Fedlmeier JE, Czarnecki D (1988) Air embolism complicating percutaneous lung biopsy. AJR Am J Roentgenol 150:555–556
Kodama F, Ogawa T, Hashimoto M, Tanabe Y, Suto Y, Kato T (1999) Fatal air embolism as a complication of CT-guided needle biopsy of the lung. J Comput Assist Tomogr 23:949–951
Hare SS, Gupta A, Goncalves AT, Souza CA, Matzinger F, Seely JM (2011) Systemic arterial air embolism after percutaneous lung biopsy. Clin Radiol 66:589–596
Ibukuro K, Tanaka R, Takeguchi T, Fukuda H, Abe S, Tobe K (2009) Air embolism and needle track implantation complicating CT-guided percutaneous thoracic biopsy: single-institution experience. AJR Am J Roentgenol 193:W430–W436
Murad MH, Sultan S, Haffar S, Bazerbachi F (2018) Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 23:60–63
Stijnen T, Hamza TH, Özdemir P (2010) Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 29:3046–3067
Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ (2014) In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 67:897–903
Roy DC, Mehrotra ML (1974) An evaluation of percutaneous trephine biopsy in the diagnosis of lung diseases. Am Rev Respir Dis 110:133–136
Kuo HL, Cheng L, Chung TJ (2010) Systemic air embolism detected during percutaneous transthoracic needle biopsy: report of two cases and a proposal for a routine postprocedure computed tomography scan of the aorto-cardiac region. Clin Imaging 34:53–56
Lucidarme O, Howarth N, Finet JF, Grenier PA (1998) Intrapulmonary lesions: percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. Radiology 207:759–765
Pando Sandoval A, Ariza Prota MA, Garcia Clemente M, Prieto A, Fole Vazquez D, Casan P (2015) Air embolism: a complication of computed tomography-guided transthoracic needle biopsy. Respirol Case Rep 3:48–50
Azrumelashvili T, Mizandari M, Dundua T (2016) Imaging guided percutaneal core biopsy of pulmonary and pleural masses - technique and complications. Georgian Med News 250:25–33
Sinner WN (1979) Pulmonary neoplasms diagnosed with transthoracic needle biopsy. Cancer 43:1533–1540
Sun C, Bian J, Lai S, Li X (2015) Systemic air embolism as a complication of CT-guided percutaneous core needle lung biopsy: a case report and review of the literature. Exp Ther Med 10:1157–1160
Szlezak P, Śrutek E, Gorycki T, Kowalewski J, Studniarek M (2014) Core-needle biopsy under CT fluoroscopy guidance and fine-needle aspiration cytology: comparison of diagnostic yield in the diagnosis of lung and mediastinum tumors. Analysis of frequency and types of complications. Pol J Radiol 79:175–180
Takeshita J, Masago K, Kato R et al (2015) CT-guided fine-needle aspiration and core needle biopsies of pulmonary lesions: a single-center experience with 750 biopsies in Japan. AJR Am J Roentgenol 204:29–34
Monnin-Bares V, Chassagnon G, Vernhet-Kovacsik H et al (2019) Systemic air embolism depicted on systematic whole thoracic CT acquisition after percutaneous lung biopsy: incidence and risk factors. Eur J Radiol 117:26–32
Liu SH, Fu Q, Yu HL et al (2020) A retrospective analysis of the risk factors associated with systemic air embolism following percutaneous lung biopsy. Exp Ther Med 19:347–352
Aberle DR, Gamsu G, Golden JA (1987) Fatal systemic arterial air embolism following lung needle aspiration. Radiology 165:351–353
Al-Ali WM, Browne T, Jones R (2012) A case of cranial air embolism after transthoracic lung biopsy. Am J Respir Crit Care Med 186:1193–1195
April D, Sandow T, Scheibal J, Devun D, Kay D (2017) Left atrial air embolism following computed tomography–guided lung biopsy. Ochsner J 17:141–143
Arnold BW, Zwiebel WJ (2002) Percutaneous transthoracic needle biopsy complicated by air embolism. AJR Am J Roentgenol 178:1400–1402
Beliaev AM, Milne D, Sames C, O’Brien B, Ramanathan T (2019) Massive arteriovenous air embolism after computed tomography-guided lung tumour biopsy. ANZ J Surg 89:434–436
Bhatia S (2009) Systemic air embolism following CT-guided lung biopsy. J Vasc Interv Radiol 20:709–711
Bou-Assaly W, Pernicano P, Hoeffner E (2010) Systemic air embolism after transthoracic lung biopsy: a case report and review of literature. World J Radiol 2:193–196
Kooblall M, Lane SJ, Moloney E (2015) ‘Life saving positioning’ in patients with air embolism. Ir Med J 108:318
Chakravarti R, Singh V, Isaac R, John MJ (2004) Fatal paradoxical pulmonary air embolism complicating percutaneous computed tomography-guided needle biopsy of the lung. Australas Radiol 48:204–206
Chang HC, Yang MC (2018) Systemic air embolism after percutaneous computed tomography-guided lung biopsy due to a kink in the coaxial biopsy system: a case report. BMC Med Imaging 18:1. https://doi.org/10.1186/s12880-018-0245-9
Chang M, Marshall J (2012) Therapeutic hypothermia for acute air embolic stroke. West J Emerg Med 13:111–113
Iwuagwu OC, Felix EO (2010) Left ventricular and coronary air embolism complicating a routine CT-guided cavitating lung mass biopsy: a case report. Res J Med Sci 4:248–251
Dietrich A, Vargas A, Smith DE, Domenech A (2017) Intraventricular air embolism complicating computed tomography-guided pulmonary aspiration biopsy. Arch Bronconeumol 53:348–349
El Homsi M, Haydar A, Dughayli J, Al-Kutoubi A (2019) Trans-catheter aspiration of systemic air embolism causing cardiac compromise during CT-guided lung biopsy, a potentially lifesaving maneuver. Cardiovasc Intervent Radiol 42:150–153
Fiore L, Frenk NE, Martins GLP, Viana PCC, de Menezes MR (2017) Systemic air embolism after percutaneous lung biopsy: a manageable complication. J Radiol Case Rep 11:6–14
Tomabechi M, Kato K, Sone M et al (2008) Cerebral air embolism treated with hyperbaric oxygen therapy following percutaneous transthoracic computed tomography-guided needle biopsy of the lung. Radiat Med 26:379–383
Franke M, Reinhardt HC, Von Bergwelt-Baildon M, Bangard C (2014) Massive air embolism after lung biopsy. Circulation 129:1046–1047
Galvis JM, Nunley DR, Zheyi T, Dinglasan LAV (2017) Left ventricle and systemic air embolism after percutaneous lung biopsy. Respir Med Case Rep 22:206–208
Ghafoori M, Varedi P (2008) Systemic air embolism after percutaneous transthorasic needle biopsy of the lung. Emerg Radiol 15:353–356
Hirasawa S, Hirasawa H, Taketomi-Takahashi A et al (2008) Air embolism detected during computed tomography fluoroscopically guided transthoracic needle biopsy. Cardiovasc Intervent Radiol 31:219–221
Hsi DH, Thompson TN, Fruchter A, Collins MS, Lieberg OU, Boepple H (2008) Simultaneous coronary and cerebral air embolism after CT-guided core needle biopsy of the lung. Tex Heart Inst J 35:472–474
Hung WH, Chang CC, Ho SY, Liao CY, Wang BY (2015) Systemic air embolism causing acute stroke and myocardial infarction after percutaneous transthoracic lung biopsy-a case report. J Cardiothorac Surg 10:121. https://doi.org/10.1186/s13019-015-0329-3
Ialongo P, Ciarpaglini L, Tinti MD, Suadoni MN, Cardillo G (2017) Systemic air embolism as a complication of percutaneous computed tomography guided transthoracic lung biopsy. Ann R Coll Surg Engl 99:e174–e176
Ishikawa Y, Matsuguma H, Nakahara R, Ui A, Suzuki H, Yokoi K (2009) Arterial air embolism: a rare but life-threatening complication of percutaneous needle biopsy of the lung. Ann Thorac Surg 87:1622. https://doi.org/10.1016/j.athoracsur.2008.08.035
Karnatovskaia LV, Lee AS, Dababneh H, Lin A, Festic E (2012) Stress-induced cardiomyopathy complicating a stroke caused by an air embolism. J Bronchology Interv Pulmonol 19:224–227
Kau T, Rabitsch E, Celedin S, Habernig SM, Weber JR, Hausegger KA (2008) When coughing can cause stroke--a case-based update on cerebral air embolism complicating biopsy of the lung. Cardiovasc Intervent Radiol 31:848–853
Kawaji T, Shiomi H, Togashi Y et al (2012) Coronary air embolism and cardiogenic shock during computed tomography-guided needle biopsy of the lung. Circulation 126:e195–e197
Kazimirko DN, Beam WB, Saleh K, Patel AM (2016) Beware of positive pressure: coronary artery air embolism following percutaneous lung biopsy. Radiol Case Rep 11:344–347
Cianci P, Posin JP, Shimshak RR, Singzon J (1987) Air embolism complicating percutaneous thin needle biopsy of lung. Chest 92:749–751
Khalid F, Rehman S, AbdulRahman R, Gupta S (2018) Fatal air embolism following local anaesthetisation: does needle size matter? BMJ Case Rep 2018:bcr2017222254. https://doi.org/10.1136/bcr-2017-222254
Khatri S (1997) Cerebral artery gas embolism (CAGE) following fine needle aspiration biopsy of the lung. Aust N Z J Med 27:338. https://doi.org/10.1111/j.1445-5994.1997.tb01989.x
Reguero Llorente E, Alonso García E (2019) Arterial air embolism after percutaneous lung biopsy. Radiologia 61:269–270
Kogut MJ, Linville RM, Bastawrous S, Padia SA, Maki JH, Bhargava P (2012) Systemic air embolization during percutaneous transthoracic needle biopsy: imaging findings, management strategies, and review of the literature. Clin Pulm Med 19:188–190
Kok HK, Leong S, Salati U, Torreggiani WC, Govender P (2013) Left atrial and systemic air embolism after lung biopsy: importance of treatment positioning. J Vasc Interv Radiol 24:1587–1588
Lang D, Reinelt V, Horner A et al (2018) Complications of CT-guided transthoracic lung biopsy: a short report on current literature and a case of systemic air embolism. Wien Klin Wochenschr 130:288–292
Lattin G Jr, O'Brien W Sr, McCrary B, Kearney P, Gover D (2006) Massive systemic air embolism treated with hyperbaric oxygen therapy following CT-guided transthoracic needle biopsy of a pulmonary nodule. J Vasc Interv Radiol 17:1355–1358
Lederer W, Schlimp CJ, Glodny B, Wiedermann FJ (2011) Air embolism during CT-guided transthoracic needle biopsy. BMJ Case Rep 2011:bcr0420114113. https://doi.org/10.1136/bcr.04.2011.4113
Lee WH (2016) Air embolization after computed tomography-guided percutaneous transthoracic needle biopsy. Soonchunhyang Med Sci 22:170–172
Mansour A, AbdelRaouf S, Qandeel M, Swaidan M (2005) Acute coronary artery air embolism following CT-guided lung biopsy. Cardiovasc Intervent Radiol 28:131–134
Matsuura H, Takaishi A, Oonishi N et al (2017) Air embolism and CT-guided lung biopsy. QJM 110:465–466
Matz S, Segal A, Nemesh L (1980) Diagnosis of air embolism to the brain by computerized axial tomography. Comput Tomogr 4:107–110
Deshmukh A, Kadavani N, Kakkar R, Desai S, Bhat GM (2019) Coronary artery air embolism complicating a CT-guided percutaneous lung biopsy. Indian J Radiol Imaging 29:81–84
Mokart D, Sarran A, Barthelemy A et al (2011) Systemic air embolism during lung biopsy. Br J Anaesth 107:277–278
Mokhlesi B, Ansaarie I, Bader M, Tareen M, Boatman J (2002) Coronary artery air embolism complicating a CT-guided transthoracic needle biopsy of the lung. Chest 121:993–996
Baker BK, Awwad EE (1988) Computed tomography of fatal cerebral air embolism following percutaneous aspiration biopsy of the lung. J Comput Assist Tomogr 12:1082–1083
Ohashi S, Endoh H, Honda T, Komura N, Satoh K (2001) Cerebral air embolism complicating percutaneous thin-needle biopsy of the lung: complete neurological recovery after hyperbaric oxygen therapy. J Anesth 15:233–236
Olgun DC, Samanci C, Ergin AS, Akman C (2015) Life-threatening complication of percutaneous transthoracic fine-needle aspiration biopsy: systemic arterial air embolism. Eurasian J Med 47:72–74
Omenaas E, Moerkve O, Thomassen L et al (1989) Cerebral air embolism after transthoracic aspiration with a 0.6 mm (23 gauge) needle. Eur Respir J 2:908–910
Ornelas E, Fernandez-Vilches S, Gallardo X, Mesquida J (2018) Massive coronary air embolism after CT-guided lung needle biopsy. Intensive Care Med 44:1748–1749
Sood B, Rezaei S, Brodribb R (2012) Iatrogenic air embolism following CT-guided lung biopsy. Appl Radiol 41:43–46
Rahman ZU, Murtaza G, Pourmorteza M et al (2016) Cardiac arrest as a consequence of air embolism: a case report and literature review. Case Rep Med 2016:8236845. https://doi.org/10.1155/2016/8236845
Ramaswamy R, Narsinh KH, Tuan A, Kinney TB (2014) Systemic air embolism following percutaneous lung biopsy. Semin Intervent Radiol 31:375–377
Regge D, Gallo T, Galli J, Bertinetti A, Gallino C, Scappaticci E (1997) Systemic arterial air embolism and tension pneumothorax: two complications of transthoracic percutaneous thin-needle biopsy in the same patient. Eur Radiol 7:173–175
Rocha RD, Azevedo AA, Falsarella PM, Rahal A, Garcia RG (2015) Cerebral air embolism during CT-guided lung biopsy. Thorax 70:1099–1100
Rott G, Boecker F (2014) Influenceable and avoidable risk factors for systemic air embolism due to percutaneous CT-guided lung biopsy: patient positioning and coaxial biopsy technique - case report, systematic literature review, and a technical note. Radiol Res Pract 2014:349062. https://doi.org/10.1155/2014/349062
Sakatani T, Amano Y, Sato J, Nagase T (2018) Air embolism after CT-guided percutaneous lung biopsy. Jpn J Clin Oncol 48:699–700
Ashizawa K, Watanabe H, Morooka H, Hayashi K (2004) Hyperbaric oxygen therapy for air embolism complicating CT-guided needle biopsy of the lung. AJR Am J Roentgenol 182:1606–1607
Shin KM, Lim JK, Kim CH (2014) Delayed presentation of cerebellar and spinal cord infarction as a complication of computed tomography-guided transthoracic lung biopsy: a case report. J Med Case Reports 8:272. https://doi.org/10.1186/1752-1947-8-272
Shroff GR, Sarraf M, Sprenkle MD, Karim RM (2011) Air embolism involving the coronary and pulmonary circulation: an unusual cause of sudden cardiac death. Circulation 124:2949–2950
Singh A, Ramanakumar A, Hannan J (2011) Simultaneous left ventricular and cerebral artery air embolism after computed tomographic-guided transthoracic needle biopsy of the lung. Tex Heart Inst J 38:424–426
Smit DR, Kleijn SA, de Voogt WG (2013) Coronary and cerebral air embolism: a rare complication of computed tomography-guided transthoracic lung biopsy. Neth Heart J 21:464–466
Pereira P (1993) A fatal case of cerebral artery gas embolism following fine needle biopsy of the lung. Med J Aust 159:755–757
Suzuki K, Ueda M, Muraga K et al (2013) An unusual cerebral air embolism developing within the posterior circulation territory after a needle lung biopsy. Intern Med 52:115–117
Yoshida R, Yoshizako T, Nakamura M et al (2018) Nonfatal air embolism complicating percutaneous CT-guided lung biopsy and VATS marking: four cases from a single institution. Clin Imaging 48:127–130
Yamamoto A, Suzuki K, Iwashita Y et al (2016) Controlled normothermia for a cerebral air embolism complicating computed tomography-guided transthoracic needle biopsy of the lung. Acute Med Surg 3:411–414
Thomas R, Thangakunam B, Cherian RA, Gupta R, Christopher DJ (2011) Cerebral air embolism complicating CT-guided trans-thoracic needle biopsy of the lung. Clin Respir J 5:e1–e3
Shi L, Zhang R, Wang Z, Zhou P (2013) Delayed cerebral air embolism complicating percutaneous needle biopsy of the lung. Am J Med Sci 345:501–503
Tsetsou S, Eeckhout E, Qanadli SD, Lachenal Y, Vingerhoets F, Michel P (2013) Non-accidental arterial cerebral air embolism: a 10-year stroke center experience. Cerebrovasc Dis 35:392–395
Um SJ, Lee SK, Doo KY et al (2009) Four cases of a cerebral air embolism complicating a percutaneous transthoracic needle biopsy. Korean J Radiol 10:81–84
Wu YF, Huang TW, Kao CC, Lee SC (2012) Air embolism complicating computed tomography-guided core needle biopsy of the lung. Interact Cardiovasc Thorac Surg 14:771–772
Viqas Z, Yar A, Yaseen M, Khalid M (2018) Cardiac arrest due to air embolism: complicating image-guided lung biopsy. Cureus 10:e3295. https://doi.org/10.7759/cureus.3295
Westcott JL (1973) Air embolism complicating percutaneous needle biopsy of the lung. Chest 63:108–110
Wong RS, Ketai L, Temes RT, Follis FM, Ashby R (1995) Air embolus complicating transthoracic percutaneous needle biopsy. Ann Thorac Surg 59:1010–1011
Tavare AN, Patel A, Saini A, Creer DD, Hare SS (2018) Systemic air embolism as a complication of percutaneous lung biopsy. Br J Hosp Med (Lond) 79:106–107
Marchak K, Hong MJ, Schramm KM (2019) Systemic air embolism following CT-guided percutaneous core needle biopsy of the lung: case report and review of the literature. Semin Intervent Radiol 36:68–71
Hellinger L, Keppler A, Schoeppenthau H, Perras J, Bender R (2019) Hyperbaric oxygen therapy for iatrogenic arterial gas embolism after CT-guided lung biopsy : a case report. Anaesthesist 68:456–460
Warren S, Somers A, Chambers B, Gardner K (2019) A case study: percutaneous lung biopsy and symptomatic arterial air embolus. J Radiol Nurs 38:174–176
Oliva RA, de Miguel DJ, Puente ML, de la Torre FJ (2019) Arterial gas embolism: a rare complication of core needle biopsy in the diagnosis of solitary pulmonary nodule. Arch Bronconeumol 55:492–493
Abid H, Kumar A, Siddiqui N, Kramer B (2019) Systemic air embolism following computed tomography-guided lung biopsy. Cureus 11:e5408. https://doi.org/10.7759/cureus.5408
Muth CM, Shank ES (2000) Gas embolism. N Engl J Med 342:476–482
Coulter TD, Wiedemann HP (2000) Gas embolism. N Engl J Med 342:2000. https://doi.org/10.1056/NEJM200006293422617
Balsara ZN, Burks DD (2007) Hyperbaric oxygen therapy for arterial air embolism. AJR Am J Roentgenol 188:W98–W98
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Soon Ho Yoon.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise (Hyunsook Hong).
Informed consent
Written informed consent was not required because this study is a systematic review and pooled analysis of the literature.
Ethical approval
Institutional Review Board approval was not required because this study is a systematic review and pooled analysis of the literature.
Study subjects or cohorts overlap
This study is a systematic review of the literature and pooled analysis of all their patients.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J.H., Yoon, S.H., Hong, H. et al. Incidence, risk factors, and prognostic indicators of symptomatic air embolism after percutaneous transthoracic lung biopsy: a systematic review and pooled analysis. Eur Radiol 31, 2022–2033 (2021). https://doi.org/10.1007/s00330-020-07372-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07372-w