Abstract
Objectives
In patients with acute ischemic stroke, we aimed to investigate whether microvascular changes, as indexed by capillary transit time heterogeneity (CTH), contribute to the decline of the chance for favorable outcome over time and whether they are a predictor of an intracranial hemorrhage (ICH).
Methods
We retrospectively calculated CTH maps for 131 consecutive patients with acute ischemic stroke due to large vessel occlusion of the anterior circulation who had a relevant MRI PWI-DWI mismatch and were treated with endovascular thrombectomy (ET). Multivariable logistic regressions were conducted with favorable outcome (mRS ≤ 2 after 3 months) and occurrence of an ICH as dependent variables and the volume of mildly elevated CTH as independent variable adjusted for age, successful recanalization, hypertension, diabetes, atrial fibrillation, NIHSS score on admission, DWI lesion volume, and symptom-onset-to-treatment time (OTT).
Results
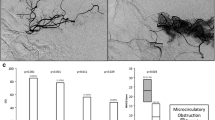
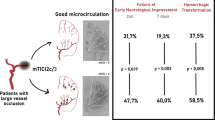
A larger volume of mildly elevated CTH was a positive predictor of favorable outcome (OR 1.17; 1.03–1.33; p = 0.019) and a negative predictor of ICH (OR 0.83; 0.73–0.96; p = 0.009). As expected, successful recanalization (OR 5.54; 1.8–17; p = 0.003), low NIHSS on admission (OR 0.9; 0.82–1.00; p = 0.045), short OTT (OR 0.96; 0.94–0.99; p = 0.006), and low DWI volume (OR 0.68; 0.49–0.94; p = 0.021) were also predictors of favorable outcome, whereas other negative predictors of ICH were atrial fibrillation (OR 2.69; 1.10–6.57; p = 0.030), high NIHSS score on admission (OR 1.10 (1.01–1.19); p = 0.030), and large DWI volume (OR 1.51; 1.17–1.19; p = 0.002).
Conclusion
An increased volume of mildly elevated CTH is a positive predictor of favorable outcome and a negative predictor for ICH in patients with acute ischemic stroke and mismatch undergoing ET.
Key Points
• The classification of potentially salvageable tissue and infarct core based on traditional net perfusion parameters (as Tmax or CBF) does not account for the microvascular distribution of blood.
• However, the microvascular distribution of blood, as indexed by the capillary transit time heterogeneity (CTH), directly affects the availability of oxygen within the hypoperfused tissue and should therefore be respected in acute ischemic stroke imaging.
• In our study, mildly elevated CTH is found to be a positive predictor for a favorable clinical outcome and a negative predictor for the occurrence of an intracranial hemorrhage in patients with acute ischemic stroke and homogenous mismatch who underwent ET.


Similar content being viewed by others
Abbreviations
- CBF:
-
Cerebral blood flow
- CBV:
-
Cerebral blood volume
- CTH:
-
Capillary transit time heterogeneity
- ET:
-
Endovascular thrombectomy
- ICA:
-
Internal carotid artery
- LVO:
-
Large vessel occlusion
- MCA:
-
Middle cerebral artery
- mRS:
-
Modified Rankin scale
- MTT:
-
Mean transit time
- NECT:
-
Non-enhanced CT imaging
- NIHSS:
-
National Institute of Health Stroke Scale
- OEF:
-
Oxygen extraction fraction
- OTT:
-
Symptom-onset-to-treatment time
References
Goyal M, Menon BK, von Zwam WH et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731
Nogueira RG, Jadhav AP, Haussen DC et al (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378:11–21
Albers GW, Marks MP, Kemp S et al (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378:708–718
Lansberg MG, Straka M, Kemp S et al (2012) MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 10:860–867
Campbell BC, Christensen S, Levi CR et al (2011) Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 42(12):3435–3440
d’Esterre CD, Boesen ME, Ahn SH et al (2015) Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke 46(12):3390–3397
Wu O, Østergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG (2003) Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 50(1):164–174
Calamante F, Christensen S, Desmond PM, Østergaard L, Davis SM, Connelly A (2010) The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke 41(6):1169–1174
Campbell BC, Mitchell PJ, Kleinig TJ et al (2015) Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372:1009–1018
Saver JL, Goyal M, Bonafe A et al (2015) Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372:2285–2295
Saver JL, Goyal M, van der Lugt A et al (2016) Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 316:1279–1289
Mundiyanapurath S, Diatschuk S, Loebel S et al (2017) Outcome of patients with proximal vessel occlusion of the anterior circulation and DWI-PWI mismatch is time-dependent. Eur J Radiol 91:82–87
Østergaard L, Jespersen SN, Mouridsen K et al (2013) The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab 33(5):635–648
Astrup J, Siesjö BK, Symon L (1981) Thresholds in cerebral ischemia - the ischemic penumbra. Stroke 12:723–725
Jespersen SN, Østergaard L (2012) The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 32(2):264–277
Pries AR, Neuhaus D, Gaehtgens P (1992) Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol 263:H1770–H1778
Engedal TS, Hjort N, Hougaard KD et al (2017) Transit time homogenization in ischemic stroke - a novel biomarker of penumbral microvascular failure? J Cereb Blood Flow Metab 38(11):2006–2020
Mouridsen K, Friston K, Hjort N, Gyldensted L, Østergaard L, Kiebel S (2006) Bayesian estimation of cerebral perfusion using a physiological model of microvasculature. Neuroimage 33(2):570–579
Mouridsen K, Hansen MB, Østergaard L, Jespersen SN (2014) Reliable estimation of capillary transit time distributions using DSC-MRI. J Cereb Blood Flow Metab 34(9):1511–1521
Schumann P, Touzani O, Young AR, Morello R, Baron JC, MacKenzie ET (1998) Evaluation of the ratio of cerebral blood flow to cerebral blood volume as an index of local cerebral perfusion pressure. Brain 121(7):1369–1379
Rasmussen PM, Jespersen SN, Østergaard L (2015) The effects of transit time heterogeneity on brain oxygenation during rest and functional activation. J Cereb Blood Flow Metab 35(3):432–442
Potreck A, Seker F, Hoffmann A et al (2017) A novel method to assess pial collateralization from stroke perfusion MRI: subdividing Tmax into anatomical compartments. Eur Radiol 27(2):618–626
Mundiyanapurath S, Ringleb PA, Diatschuk S et al (2016) Capillary transit time heterogeneity is associated with modified Rankin scale score at discharge in patients with bilateral high grade internal carotid artery stenosis. PLoS One 11(6):e0158148
Smith AM, Grandin CB, Duprez T, Mataigne F, Cosnard G (2000) Whole brain quantitative CBF, CBV, and MTT measurements using MRI bolus tracking: implementation and application to data acquired from hyperacute stroke patients. J Magn Reson Imaging 12(3):400–410
Derdeyn CP, Videen TO, Yundt KD et al (2002) Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 125:595–607
Baron JC, Jones T (2012) Oxygen metabolism, oxygen extraction and positron emission tomography: historical perspective and impact on basic and clinical neuroscience. Neuroimage 61(2):492–504
Angleys H, Østergaard L, Jespersen SN (2015) The effects of capillary transit time heterogeneity (CTH) on brain oxygenation. J Cereb Blood Flow Metab 35(5):806–817
Liu S, Connor J, Peterson S, Shuttleworth CW, Liu KJ (2002) Direct visualization of trapped erythrocytes in rat brain after focal ischemia and reperfusion. J Cereb Flow Metab 10:1222–1230
Zhang ZG, Chopp M, Goussev A et al (1999) Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats. J Neurosci 19(24):10898–10907
Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T (2009) Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 15(9):1031–1037
Peppiatt CM, Howarth C, Mobbs P, Attwell D (2006) Bidirectional control of CNS capillary diameter by pericytes. Nature 443:700–704
Kwon I, Kim EH, del Zoppo GJ, Heo JH (2009) Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res 87(3):668–676
Ames A 3rd, Wright LW, Kowada M, Thurston JM, Majors G (1968) Cerebral ischemia, II: the no-reflow phenomenon. Am J Pathol 52:437–453
Srinivasan VJ, Mandeville ET, Can A et al (2013) Multiparametric, longitudinal optical coherence tomography imaging reveals acute injury and chronic recovery in experimental ischemic stroke. PLoS One 8(8):e71478
Goyal M, Menon BK, Almekhlafi MA, Demchuk A, Hill MD (2017) The need for better data on patients with acute stroke who are not treated because of unfavorable imaging. AJNR Am J Neuroradiol 38(3):424–425
Federau C, Mlynash M, Christensen S et al (2016) Evolution of volume and signal intensity on fluid-attenuated inversion recovery MR images after endovascular stroke therapy. Radiology 280(1):184–192
Krongold M, Almekhlafi MA, Demchuk AM, Coutts SB, Frayne R, Eilaghi A (2015) Final infarct volume estimation on 1-week follow-up MR imaging is feasible and is dependent on recanalization status. Neuroimage Clin 7:1–6
Larsson HBW, Vestergaard MB, Lindberg U, Iversen HK, Cramer SP (2017) Brain capillary transit time heterogeneity in healthy volunteers measured by dynamic contrast-enhanced T1 -weighted perfusion MRI. J Magn Reson Imaging 45(6):1809–1820
Kidwell CS, Jahan R, Gornbein J et al (2013) A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 368:91
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is S. Mundiyanapurath.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
The study population differs by only eight patients (in whom PWI quality was not sufficient for CTH analysis) from the cohort in a preceding work by Mundiyanapurath et al [7]. While this preceding work concentrated on the effect of perfusion-diffusion mismatch early or late after symptom onset, we now investigated the role of the perfusion index CTH in regard to the prediction of a favorable outcome and the occurrence of an intracranial hemorrhage after endovascular thrombectomy.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Potreck, A., Loebel, S., Pfaff, J. et al. Increased volumes of mildly elevated capillary transit time heterogeneity positively predict favorable outcome and negatively predict intracranial hemorrhage in acute ischemic stroke with large vessel occlusion. Eur Radiol 29, 3523–3532 (2019). https://doi.org/10.1007/s00330-019-06064-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06064-4