Abstract
Objective
MRI is crucial for the classification of hepatocellular adenomas (HCA) into subtypes. Our objective was to review and increase MRI criteria for subtype classification and define the limits.
Methods
Pathological and radiological data of 116 HCAs were retrospectively analyzed to investigate MRI features of HCA pathological subtypes. Risk for complication was also evaluated with regard to subtype and tumor size.
Results
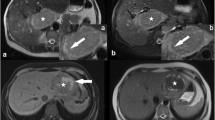
38/43 (88%) HNF1α-mutated HCAs (H-HCAs) were discriminated by (i) fatty component (homogeneous or heterogeneous) and (ii) hypovascular pattern, with a sensitivity of 88% and a specificity of 97%. 51/58 (88%) inflammatory HCAs (IHCAs) displayed features of sinusoidal dilatation (SD) including three different patterns (global SD, atoll sign, and a new “crescent sign” corresponding to a partial peripheral rim, hyperintense on T2W and/or arterial phase with persistent delayed enhancement). Sensitivity was 88% and specificity 100%. However, some HCA remained unclassifiable by MRI: HCA remodeled by necrotic/hemorrhagic changes covering > 50% of the lesion, H-HCAs without steatosis, IHCAs without SD, β-catenin-mutated and unclassified HCAs. Regarding malignant transformation (5/116) and bleeding (24/116), none was observed when the HCA diameter was smaller than 5.2 cm and 4.2 cm, respectively.
Conclusion
Based on the largest series evaluated until now, we identified several non-described MRI features and propose new highly sensitive and specific MRI criteria. With the addition of these new features, 88% of the two main HCA subtypes could be identified.
Key Points
• HNF1α-mutated hepatocellular adenomas (H-HCA) are characterized by the presence of fat and hypovascular pattern in MRI.
• Inflammatory hepatocellular adenomas (I-HCA) are characterized by different patterns translating sinusoidal dilatation including the newly described crescent sign.
• No MRI specific pattern was identified for β-catenin-mutated HCA (b-HCA).





Similar content being viewed by others
Abbreviations
- Ass1:
-
Argininosuccinate synthase 1
- AUROC:
-
Area under the receiver operating characteristic
- b-HCA:
-
β-catenin-mutated HCA
- b-IHCA:
-
β-catenin-mutated inflammatory HCA
- CRP:
-
C-reactive protein
- FNH:
-
Focal nodular hyperplasia
- H-HCA:
-
HNF1α-mutated HCAs
- HBP:
-
Hepatobiliary phase
- HCA:
-
Hepatocellular adenoma
- HCC:
-
Hepatocellular carcinoma
- IHCA:
-
Inflammatory HCA
- LFABP:
-
Liver fatty acid-binding protein
- MRI:
-
Magnetic resonance imaging
- SD:
-
Sinusoidal dilatation
- T2W:
-
T2-weighted sequence
- UHCA:
-
Unclassified HCA
References
Rooks JB, Ory HW, Ishak KG et al (1979) Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 242:644–648
Nault JC, Bioulac-Sage P, Zucman-Rossi J (2013) Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology 144:888–902
Bioulac-Sage P, Rebouissou S, Thomas C et al (2007) Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 46:740–748
Bioulac-Sage P, Balabaud C, Zucman-Rossi J (2010) Subtype classification of hepatocellular adenoma. Dig Surg 27:39–45
Paradis V, Benzekri A, Dargère D et al (2004) Telangiectatic focal nodular hyperplasia: a variant of hepatocellular adenoma. Gastroenterology 126:1323–1329
Bioulac-Sage P, Rebouissou S, Sa Cunha A et al (2005) Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology 128:1211–1218
Rebouissou S, Amessou M, Couchy G et al (2009) Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 457:200–204
Pilati C, Amessou M, Bihl MP et al (2011) Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J Exp Med 208:1359–1366
Nault JC, Fabre M, Couchy G et al (2012) GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J Hepatol 56:184–191
Bioulac-Sage P, Laumonier H, Couchy G et al (2009) Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 50:481–489
Zucman-Rossi J, Jeannot E, Nhieu JT et al (2006) Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 43:515–524
Stoot JH, Coelen RJ, De Jong MC, Dejong CH (2010) Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 12:509–522
Van der Borght S, Libbrecht L, Katoonizadeh A et al (2007) Nuclear beta-catenin staining and absence of steatosis are indicators of hepatocellular adenomas with an increased risk of malignancy. Histopathology 51:855–856
Nault JC, Couchy G, Balabaud C et al (2017) Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology 152:880–894 e6
Henriet E, Abou Hammoud A, Dupuy JW et al (2017) Argininosuccinate synthase 1 (ASS1): a marker of unclassified hepatocellular adenoma and high bleeding risk. Hepatology 66:2016–2028
Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H (2008) Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology 48:808–818
Ronot M, Bahrami S, Calderaro J et al (2011) Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 53:1182–1191
van Aalten SM, Thomeer MG, Terkivatan T et al (2011) Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology 261:172–181
Grazioli L, Olivetti L, Mazza G, Bondioni MP (2013) MR imaging of hepatocellular adenomas and differential diagnosis dilemma. Int J Hepatol 2013:374170
Wu JS, Hochman MG (2009) Soft-tissue tumors and tumorlike lesions: a systematic imaging approach. Radiology 253:297–316
Salaria SN, Graham RP, Aishima S, Mounajjed T, Yeh MM, Torbenson MS (2015) Primary hepatic tumors with myxoid change: morphologically unique hepatic adenomas and hepatocellular carcinomas. Am J Surg Pathol 39:318–324
Merkle EM, Nelson RC (2006) Dual gradient-echo in-phase and opposed-phase hepatic MR imaging: a useful tool for evaluating more than fatty infiltration or fatty sparing. Radiographics 26:1409–1418
Bieze M, Phoa SS, Verheij J, van Lienden KP, van Gulik TM (2014) Risk factors for bleeding in hepatocellular adenoma. Br J Surg 101:847–855
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Tse JR, Naini BV, Lu DS, Raman SS (2016) Qualitative and quantitative gadoxetic acid-enhanced MR imaging helps subtype hepatocellular adenomas. Radiology 279:118–127
Ishii M, Kazaoka J, Fukushima J et al (2015) A case of beta-catenin-positive hepatocellular adenoma with MR imaging sign of diffuse intratumoral fat deposition. Abdom Imaging 40:1487–1491
Yoneda N, Matsui O, Kitao A et al (2012) Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol 30:777–782
Ba-Ssalamah A, Antunes C, Feier D et al (2015) Morphologic and molecular features of hepatocellular adenoma with gadoxetic acid-enhanced MR imaging. Radiology 277:104–113
Guo Y, Li W, Cai W, Zhang Y, Fang Y, Hong G (2017) Diagnostic value of gadoxetic acid-enhanced MR imaging to distinguish HCA and its subtype from FNH: a systematic review. Int J Med Sci 14:668–674
Belghiti J, Cauchy F, Paradis V, Vilgrain V (2014) Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol 11:737–749
Dokmak S, Paradis V, Vilgrain V et al (2009) A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 137:1698–1705
van Aalten SM, de Man RA, IJzermans JN, Terkivatan T (2012) Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg 99:911–916
Bioulac-Sage P, Sempoux C, Possenti L et al (2013) Pathological diagnosis of hepatocellular cellular adenoma according to the clinical context. Int J Hepatol 2013:253261
Sempoux C, Paradis V, Komuta M et al (2015) Hepatocellular nodules expressing markers of hepatocellular adenomas in Budd-Chiari syndrome and other rare hepatic vascular disorders. J Hepatol 63:1173–1180
Agnello F, Ronot M, Valla DC, Sinkus R, Van Beers BE, Vilgrain V (2012) High-b-value diffusion-weighted MR imaging of benign hepatocellular lesions: quantitative and qualitative analysis. Radiology 262:511–519
Zarghampour M, Fouladi DF, Pandey A et al (2018) Utility of volumetric contrast-enhanced and diffusion-weighted MRI in differentiating between common primary hypervascular liver tumors. J Magn Reson Imaging. https://doi.org/10.1002/jmri.26032
Reizine E, Amaddeo G, Pigneur F et al (2018) Quantitative correlation between uptake of Gd-BOPTA on hepatobiliary phase and tumor molecular features in patients with benign hepatocellular lesions. Eur Radiol. https://doi.org/10.1007/s00330-018-5438-7
Acknowledgments
We thank Professors Jean Saric and Laurence Chiche (surgeons); Brigitte Le Bail and Dr. Claire Castain (liver pathologists); and Florent Maire for their clinical contributions to this study.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Frulio Nora.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects (30 cases) have been previously reported in Hepatology 2008 by Laumonier et al—Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Rights and permissions
About this article
Cite this article
Bise, S., Frulio, N., Hocquelet, A. et al. New MRI features improve subtype classification of hepatocellular adenoma. Eur Radiol 29, 2436–2447 (2019). https://doi.org/10.1007/s00330-018-5784-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5784-5