Abstract
Objectives
The aim of our study was to evaluate the tumour volume doubling time (TVDT) of molecular breast cancer subtypes by serial ultrasound (US).
Methods
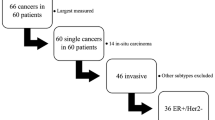
Sixty-six patients (mean age, 50 years; range, 29–78 years) with invasive breast cancer underwent initial and follow-up breast US examinations (at least three months apart) with no intervention. TVDT was determined using the tumours’ greatest dimensions in two orthogonal planes. The results were compared with clinical, imaging, and tumour variables and molecular subtypes (oestrogen receptor [ER]-positive, human epidermal growth factor receptor 2 [HER2]-positive, and triple negative) using a multiple linear regression analysis.
Results
TVDT exhibited a wide range (46–825 days; median, 141 days) with an overall mean of 193 ± 141 days and mean values of 241 ± 166 days for ER-positive tumours (n = 37), 162 ± 60 days for HER2-positive tumours (n = 12), and 103 ± 43 days for triple-negative tumours (n = 17) (P < 0.0001). In a multivariate regression analysis, compared to other features, only the different molecular breast cancer subtypes showed significant difference in TVDT (P < 0.0001).
Conclusions
TVDT differed significantly among the three molecular breast cancer subtypes, with the triple-negative tumours showing the fastest growth.
Key Points
• Knowledge of tumour volume doubling time provides clues for improving screening.
• TVDT assessed by serial US differed significantly between breast cancer subtypes.
• Triple-negative tumours had 2.4-fold shorter TVDT compared to ER-positive tumours.
• Tumours classified as BI-RADS 3 had shorter TVDT than BI-RADS 4.





Similar content being viewed by others
Abbreviations
- TVDT :
-
Tumour volume doubling time
- ER :
-
oestrogen receptor
- PR :
-
progesterone receptor
- HER2 :
-
human epidermal growth factor receptor 2
- IHC :
-
immunohistochemistry
- BI-RADS :
-
Breast Imaging Reporting and Data System
References
Friberg S, Mattson S (1997) On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol 65:284–297
Peer PG, van Dijck JA, Hendriks JH, Holland R, Verbeek AL (1993) Age-dependent growth rate of primary breast cancer. Cancer 71:3547–3551
von Fournier D, Weber E, Hoeffken W, Bauer M, Kubli F, Barth V (1980) Growth rate of 147 mammary carcinomas. Cancer 45:2198–2207
Robertson JF, Caseldine J, Winfield S (1987) Breast mammography and tumour volume. Br J Cancer 56:902
Millet I, Bouic-Pages E, Hoa D, Azria D, Taourel P (2011) Growth of breast cancer recurrences assessed by consecutive MRI. BMC Cancer 11:155
Madeleine MA, Tilanus-Linthorst MM, Obdeijn IM et al (2007) BRCA1 mutation and young age predict fast breast cancer growth in the Dutch, United Kingdom, and Canadian magnetic resonance imaging screening trials. Clin Cancer Res 13:7357–7362
Bosch AM, Kessels AG, Beets GL et al (2003) Preoperative estimation of the pathological breast tumour size by physical examination, mammography and ultrasound: a prospective study on 105 invasive tumours. Eur J Radiol 48:285–292
Moon HJ, Kim EK, Kwak JY, Yoon JH, Kim MJ (2011) Interval growth of probably benign breast lesions on follow-up ultrasound: how can these be managed? Eur Radiol 21:908–918
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98:10869–10874
Carey LA, Perou CM, Livasy CA et al (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492–2502
Lin NU, Vanderplas A, Hughes ME et al (2012) Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118:5463–5472
de Ronde JJ, Hannemann J, Halfwerk H et al (2010) Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat 119:119–126
Dawson SJ, Duffy SW, Blows FM et al (2009) Molecular characteristics of screen-detected vs symptomatic breast cancers and their impact on survival. Br J Cancer 101:1338–1344
Dogan BE, Gonzalez-Angulo AM, Gilcrease M, Dryden MJ, Yang WT (2010) Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am J Roentgenol 194:1160–1166
Ko ES, Lee BH, Kim HA, Noh WC, Kim MS, Lee SA (2010) Triple-negative breast cancer: correlation between imaging and pathological findings. Eur Radiol 20:1111–1117
Boisserie-Lacroix M, Macgrogan G, Debled M et al (2013) Triple-negative breast cancers: associations between imaging and pathological findings for triple-negative tumors compared with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers. Oncologist 18:802–811
Mendelson EB, Baum JK, Berg WA, Merritt CRB, Rubin E (2003) Breast Imaging Reporting and Data System, BI-RADS: Ultrasound, 1st edn. American College of Radiology, Reston
Tilanus-Linthorst MM, Kriege M, Boetes C et al (2005) Hereditary breast cancer growth rates and its impact on screening policy. Eur J Cancer 41:1610–1617
Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S (2010) American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 6:195–197
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Cheang MC, Chia SK, Voduc D et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Turkoz FP, Solak M, Petekkaya I et al (2013) Association between common risk factors and molecular subtypes in breast cancer patients. Breast 22:344–350
Eklund M, Esserman LJ (2013) Screening: biology dictates the fate of young women with breast cancer. Nat Rev Clin Oncol 10:673–675
de Melo Gagliato D, Gonzalez-Angulo AM, Lei X et al (2014) Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol(Jan 27)
Park S, Koo JS, Kim MS et al (2012) Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 21:50–57
Lee JA, Kim KI, Bae JW et al (2010) Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat 123:177–178
Norton L (1988) A Gompertzian model of human breast cancer growth. Cancer Res 48(24 Pt 1):7067–7071
Demicheli R, Terenziani M, Valagussa P, Moliterni A, Zambetti M, Bonadonna G (1994) Local recurrences following mastectomy: support for the concept of tumor dormancy. J Natl Cancer Inst 86:45–48
Stavros AT (2003) Breast ultrasound. Lippincott Williams & Wilkins, Philadelphia, PA, pp 490–491
Berg WA, Cosgrove DO, Doré CJ et al (2012) Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 262:435–449
Cho N, Jang M, Lyou CY, Park JS, Choi HY, Moon WK (2012) Distinguishing benign from malignant masses at breast US: combined US elastography and color doppler US–influence on radiologist accuracy. Radiology 262:80–90
Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S (2008) Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res 10:R41
Kelly KM, Dean J, Lee SJ, Comulada WS (2010) Breast cancer detection: radiologists’ performance using mammography with and without automated whole-breast ultrasound. Eur Radiol 20:2557–2564
Kuroishi T, Tominaga S, Morimoto T et al (1990) Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Jpn J Cancer Res 81:454–462
Acknowledgments
The scientific guarantor of this publication is Woo Kyung Moon. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2012R1A2A1A01010846). No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, E.B., Chang, J.M., Seo, M. et al. Tumour volume doubling time of molecular breast cancer subtypes assessed by serial breast ultrasound. Eur Radiol 24, 2227–2235 (2014). https://doi.org/10.1007/s00330-014-3256-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3256-0