Abstract
Objective
To compare the use of diffusion-weighted MR imaging (DWI) and 18F-FDG PET/CT to predict pathological complete response (pCR) in breast cancer patients receiving neoadjuvant chemotherapy.
Methods
Thirty-four women with 34 invasive breast cancers underwent DWI and PET/CT before and after chemotherapy and before surgery. The percentage changes in the apparent diffusion coefficient (ADC) and the standardised uptake value (SUV) were calculated, and the diagnostic performances for predicting pCR were evaluated using receiver operating characteristic (ROC) curve analysis.
Results
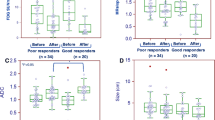
After surgery, 7/34 patients (20.6%) were found to have pCR. A z values for DWI, PET/CT and the combined use of DWI and PET/CT were 0.910, 0.873 and 0.944, respectively. The best cut-offs for differentiating pCR from non-pCR were a 54.9% increase in the ADC and a 63.9% decrease in the SUV. DWI showed 100% (7/7) sensitivity and 70.4% (19/27) specificity and PET/CT showed 100% sensitivity and 77.8% (21/27) specificity. When DWI and PET/CT were combined, there was a trend towards improved specificity compared with DWI.
Conclusions
DWI and FDG PET/CT show similar diagnostic accuracy for predicting pCR to neoadjuvant chemotherapy in breast cancer patients. The combined use of DWI and FDG PET/CT has the potential to improve specificity in predicting pCR.
Key Points
• DWI breast MR and PET/CT show similar accuracy for predicting pathological response
• The combined use of DWI and PET/CT can potentially improve specificity
• This can assist individualised treatment in breast cancer patients receiving neoadjvant chemotherapy




Similar content being viewed by others
References
Chia S, Swain SM, Byrd DR, Mankoff DA (2008) Locally advanced and inflammatory breast cancer. J Clin Oncol 26:786–790
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778–785
Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N (2002) Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) Protocol B-18. Cancer 95:681–695
Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R (2002) Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat 72:145–152
Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kuhn T (2002) Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol 12:1711–1719
Cui Y, Zhang XP, Sun YS, Tang L, Shen L (2008) Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology 248:894–900
Kim SJ, Kim SK, Lee ES, Ro J, Kang S (2004) Predictive value of [18F]FDG PET for pathological response of breast cancer to neo-adjuvant chemotherapy. Ann Oncol 15:1352–1357
Schwarz-Dose J, Untch M, Tiling R, Sassen S, Mahner S, Kahlert S, Harbeck N, Lebeau A, Brenner W, Schwaiger M, Jaenicke F, Avril N (2009) Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol 27:535–541
Herneth AM, Guccione S, Bednarski M (2003) Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol 45:208–213
Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, Hammoud DA, Rustin GJ, Taouli B, Choyke PL (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Lyng H, Haraldseth O, Rofstad EK (2000) Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med 43:828–836
Theilmann RJ, Borders R, Trouard TP, Xia G, Outwater E, Ranger-Moore J, Gillies RJ, Stopeck A (2004) Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia 6:831–837
Park SH, Moon WK, Cho N, Song IC, Chang JM, Park IA, Han W, Noh DY (2010) Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology 257:56–63
Pickles MD, Gibbs P, Lowry M, Turnbull LW (2006) Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging 24:843–847
Sharma U, Danishad KK, Seenu V, Jagannathan NR (2009) Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed 22:104–113
Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, Meszoely I, Mayer IA, Herman CR, McManus K, Price RR, Gore JC (2007) Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging 25:1–13
Eubank WB, Mankoff DA, Schmiedl UP, Winter TC 3rd, Fisher ER, Olshen AB, Graham MM, Eary JF (1998) Imaging of oncologic patients: benefit of combined CT and FDG PET in the diagnosis of malignancy. AJR Am J Roentgenol 171:1103–1110
Rosen EL, Turkington TG, Soo MS, Baker JA, Coleman RE (2005) Detection of primary breast carcinoma with a dedicated, large-field-of-view FDG PET mammography device: initial experience. Radiology 234:527–534
Rosen EL, Eubank WB, Mankoff DA (2007) FDG PET, PET/CT, and breast cancer imaging. Radiographics 27(Suppl 1):S215–S229
Rousseau C, Devillers A, Campone M, Campion L, Ferrer L, Sagan C, Ricaud M, Bridji B, Kraeber-Bodéré F (2011) FDG PET evaluation of early axillary lymph node response to neoadjuvant chemotherapy in stage II and III breast cancer patients. Eur J Nucl Med Mol Imaging 38:1029–1036
Avril N, Sassen S, Roylance R (2009) Response to therapy in breast cancer. J Nucl Med 50(Suppl 1):55S–63S
Jones RL, Lakhani SR, Ring AE, Ashley S, Walsh G, Smith IE (2006) Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer 94:358–362
Chen JH, Feig B, Agrawal G, Yu H, Carpenter PM, Mehta RS, Nalcioglu O, Su MY (2008) MRI evaluation of pathologically complete response and residual tumours in breast cancer after neoadjuvant chemotherapy. Cancer 112:17–26
Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP (2008) Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26:814–819
Altman DG (1991) Practical statistics for medical research. Chapman and Hall, London, pp 406–407
Palumbo B, Angotti F, Marano GD (2009) Relationship between PET-FDG and MRI apparent diffusion coefficients in brain tumors. Q J Nucl Med Mol Imaging 53:17–22
Gu J, Khong PL, Wang S, Chan Q, Law W, Zhang J (2010) Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Mol Imaging Biol. doi:10.1007/s11307-010-0433-7
Beer AJ, Eiber M, Souvatzoglou M, Holzapfel K, Ganter C, Weirich G, Maurer T, Kübler H, Wester HJ, Gaa J, Krause BJ (2011) Restricted water diffusibility as measured by diffusion-weighted MR imaging and choline uptake in (11)C-choline PET/CT are correlated in pelvic lymph nodes in patients with prostate cancer. Mol Imaging Biol 13:352–361
Benard F, Romsa J, Hustinx R (2003) Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin Nucl Med 33:148–162
Witney TH, Kettunen MI, Day SE, Hu DE, Neves AA, Gallagher FA, Fulton SM, Brindle KM (2009) A comparison between radiolabeled fluorodeoxyglucose uptake and hyperpolarized 13(C)-labeled pyruvate utilization as methods for detecting tumor response to treatment. Neoplasia 11:574–582
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773–1782
Chen X, Moore MO, Lehman CD, Mankoff DA, Lawton TJ, Peacock S, Schubert EK, Livingston RB (2004) Combined use of MRI and PET to monitor response and assess residual disease for locally advanced breast cancer treated with neoadjuvant chemotherapy. Acad Radiol 11:1115–1124
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 30:96–102
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L (2001) Preoperative chemotherapy in primary operative breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19:4224–4237
Moy L, Ponzo F, Noz ME, Maguire GQ Jr, Murphy-Walcott AD, Deans AE, Kitazono MT, Travascio L, Kramer EL (2007) Improving specificity of breast MRI using prone PET and fused MRI and PET 3D volume datasets. J Nucl Med 48:528–537
Berg WA, Madsen KS, Schilling K, Tartar M, Pisano ED, Larsen LH, Narayanan D, Ozonoff A, Miller JP, Kalinyak JE (2011) Breast cancer: comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radiology 258:59–72
Hamstra DA, Chenevert TL, Moffat BA, Johnson TD, Meyer CR, Mukherji SK, Quint DJ, Gebarski SS, Fan X, Tsien CI, Lawrence TS, Junck L, Rehemtulla A, Ross BD (2005) Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci USA 102:16759–16764
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A070001) and by a grant from the Innovative Research Institute for Cell Therapy, Republic of Korea (A062260).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S.H., Moon, W.K., Cho, N. et al. Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol 22, 18–25 (2012). https://doi.org/10.1007/s00330-011-2236-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2236-x