Abstract
Brainstem damage which often indicates a critical condition is usually underestimated by trans-anterior-fontanel neurosonography (NS) owing to the far-field limitations. Instead, NS alternately scanning through the squamous suture of the temporal bones and the foramen magnum could provide a better visualization of the brainstem structures. The NS characteristics of brainstem lesions caused by various neonatal neurological disorders, such as hypoxic–ischemic encephalopathy (HIE), metabolic encephalopathy, birth trauma and bacterial meningoencephalitis, can be depicted at the acute stage. An echogenic change in the midbrain was found in patients with HIE or metabolic encephalopathy. In addition to the echogenic change, bilateral transtentorial temporal lobe herniation distorting the contour of the midbrain was observed in a patient with group B streptococcus meningoencephalitis, whereas echogenic changes at the level of the pons and/or the medulla oblongata, mainly localized in the dorsal part, could be observed in newborns with severe HIE, maple syrup urine disease or birth trauma. In this pictorial assay, we demonstrate the feasibility of NS imaging in evaluating the entire brainstem structure of critically ill neonates in the near field and illustrate the characteristic features of brainstem involvement in various neonatal neurological disorders along with computed tomography or magnetic resonance imaging correlation.
Similar content being viewed by others
Introduction
The brainstem plays a crucial role in maintaining alertness and modulating respiratory and cardiovascular activity. Brainstem damage often results in a clinical life-threatening condition. Early recognition of the brainstem involvement during the acute stage of neurological disorders in high-risk newborns is important, but the clinical manifestations of brainstem involvement can be nonspecific and are often overshadowed by concurrent medical conditions. Imaging studies may provide useful information because the topography and severity of injuries determine the subsequent neurological sequelae. However, newborns with neurological disorders are often in incubators, hemodynamically unstable, and even respirator-dependent. It is often quite difficult to transport these critically ill neonates from the neonatal intensive care unit to the radiological department for magnetic resonance (MR) imaging examination. It leaves copious room for neurosonographic (NS) imaging, thanks to its portability, as a tool for critical neonates who present with neurological manifestations [1–3]. The brainstem and upper spinal cord lesions in sick neonates are commonly poorly delineated by neurosonography and may go undetected unless the examiner knows how to use these additional NS views and is familiar with the characteristic imaging patterns of brainstem injury.
NS examination through the anterior fontanel has been widely used to demonstrate intracranial abnormalities, particularly the supratentorial lesions in newborns [1–5]. However, the anterior fontanel approach plays a rather limited role in evaluating brainstem lesions [1, 6, 7]. On the other hand, an alternative lateral approach through the squamous suture of the temporal bones may potentially provide a better visualization of the upper brainstem structures, whereas the lower brainstem structures, including the medulla oblongata, can be better delineated by an approach through the foramen magnum [5, 6, 8, 9]. Herein, we showed the feasibility of NS imaging in evaluating the entire brainstem structure of critically ill neonates in the near field through the squamous suture and foramen magnum. The NS characteristics of brainstem lesions caused by various neonatal neurological disorders, such as hypoxic–ischemic encephalopathy (HIE), metabolic encephalopathy, birth trauma and bacterial meningoencephalitis, can be depicted at the acute stage. Therefore, complete NS imaging examinations through the anterior fontanel, the squamous suture of the temporal bones and the foramen magnum can be clinically important for newborns in neonatal intensive care units as far as portability and availability are concerned.
Techniques for trans-squamous and trans-foramen magnum neurosonography
NS examinations were obtained with a real-time ultrasound imaging unit equipped with a multifrequency (5–8-MHz) transducer. The neonates were examined in a supine or a side position without sedation [2, 6]. In the supine position, the transducer was placed on the squamous suture of the temporal bones on the axial plane to display the brainstem from the upper midbrain at the level of the cerebral peduncles to the pons at the level of the fourth ventricle. To delineate the axial and sagittal views of the medulla oblongata and the upper cervical cord, the transducer was placed on the midline of the upper nuchal area to scan through the foramen magnum when the neonates were in the lateral decubitus position (Fig. 1).
Schematic presentation of the supine (a) and side (b) positions of the infant being scanned. The transducer is placed at the squamous suture of the temporal bones (near the canthomeatal line) and perpendicular to the midline of the brain to obtain trans-squamous axial images (a). In the side position, the transducer is positioned on the midline of the upper nuchal area to scan through the foramen magnum (b).
NS findings of the brainstem in normal newborns
The midbrain parenchyma and cerebral peduncles appear mainly hypoechoic on trans-squamous axial imaging (Fig. 2a). The sonographic characteristics of the midbrain topography are as follows. The aqueduct of Sylvius shows as an echogenic dot in the midline of the tectum and the substantia nigra as symmetric echogenic bands located between the crus cerebri and the tegmentum. The ambient cistern appears as an echogenic curvilinear structure surrounding the midbrain. The optic tract and the parahippocampic gyrus could also be demonstrated within the same plane. Axial imaging at the level of the pons demonstrates the hypoechoic pontine parenchyma in front of the anechoic fourth ventricle (Fig. 2b), whereas sagittal imaging through the foramen magnum shows the uniformly hypoechoic medulla oblongata and the upper cervical cord, the anechoic cisterna magna and the fourth ventricle, and the hyperechoic cerebellar vermis (Fig. 2c).
Neurosonographic (NS) images of the brainstem in a normal neonate (3 days old, gestational age 38 weeks). Trans-squamous axial imaging demonstrates the hypoechoic characteristics of the midbrain and the pons at the level of the cerebral peduncles and the fourth ventricle, respectively. Within the hypoechoic midbrain structure (a), two hyperechoic areas, the aqueduct of Sylvius (black arrow) and the substantia nigra (white arrows), are indicated. The optic tract (OT), the ambient cistern (AC) and the parahippocampic gyrus (P) can also be seen within the same plane. At the level of the pons (b), the hypoechoic pontine parenchyma and the anechoic fourth ventricle (arrow) are indicated. c Sagittal imaging through the foramen magnum shows the hypoechoic medulla oblongata and the upper cervical cord, the hyperechoic cerebellar vermis, the anechoic cisterna magna (black arrow) and the fourth ventricle (white arrow).
Hypoxic–ischemic encephalopathy
HIE, which occurs in approximately 1–2 times per 1,000 live births, is an important neurological problem in the neonatal period [10]. The hypoxic–ischemic events may, however, occur in the newborn’s antepartum, intrapartum and postpartum life. HIE may indicate a pattern of hypoxic–ischemic injury predominantly affecting the thalamus, basal ganglia and brainstem. [11, 12]. Studies based on careful clinical and pathological correlations have delineated the pattern of deep nuclear-brainstem neuronal injury in neonatal HIE as a distinct clinical entity [13, 14]. Therefore, the diffuse echogenic changes involving the basal ganglia, thalamus and mesencephalic brainstem parenchyma may represent extensive neuronal injury in these vulnerable areas that frequently occurs in newborns with severe HIE [13]. In the trans-squamous axial NS imaging, an extensive echogenic change in the midbrain was considered significant when its hyperechogenicity obliterated the echogenicity of the aqueduct of Sylvius and/or the mesencephalic contour (Fig. 3).
Neuropathological studies have demonstrated that the dorsal part of the brainstem is especially vulnerable to acute profound asphyxia in both preterm and term neonates [13]. In addition to hyperechogenic midbrain lesions, focal echogenic lesions preferentially involving the pontine tegmentum (Fig. 4) and the dorsal medulla oblongata (Fig. 5) could also be found in patients with severe HIE. The corresponding computed tomography or MR images in the same regions showed findings compatible with ischemic encephalomalacia. The diffusely echogenic midbrain with focal echogenic lesions involving the dorsal pons and the medulla oblongata may represent one extreme of the spectrum in neonatal HIE: severe and abrupt ischemic events that prevent the operation of major adaptive mechanisms to divert blood flow from the hemispheres to the vital and vulnerable deep nuclear structures in the dorsal brainstem, thereby causing predominantly ischemic lesions [13].
Severe hypoxic–ischemic encephalopathy due to sudden infant death syndrome in an 11-day-old neonate with gestational age 39 weeks. a The midsagittal NS image through the foramen magnum shows increased echogenicity preferentially at the pontine tegmentum (arrows). b The axial computed tomography scan at the level of the mid pons obtained 30 days following acute hypoxic-ischemic insult shows necrosis of the pontine tegmentum (arrows).
Severe hypoxic–ischemic encephalopathy due to umbilical cord prolapse in a premature baby with gestational age 32 weeks. Midsagittal (a) and axial (b) NS images through the foramen magnum at 17 days old show longitudinal hyperechoic lesions located at the dorsal part of the medulla oblongata (arrows). The short arrow indicates the anechoic cisterna magna (b). Midsagittal (c) and axial (d) T2-weighted magnetic resonance (MR) images (3500/90) at 41 days old show compatible high-signal intensity lesions, suggesting ischemic encephalomalacia, in the same area (black arrows).
Metabolic encephalopathy by inborn errors of metabolism
Many of the inborn errors of metabolism, including urea cycle defects, organic acidaemias, certain disorders of amino acid and fatty acid metabolism, can present with symptoms of acute metabolic encephalopathy in the neonatal period [15]. The patients with metabolic encephalopathy secondary to inborn errors of metabolism usually had an extensive hyperechoic change over the cerebral and midbrain parenchyma, suggesting diffusely edematous changes. The most characteristic metabolic encephalopathy involving the brainstem is maple syrup urine disease (MSUD), in which the brainstem involved shows specific focal echogenic changes at the pontine tegmentum (Fig. 6) and the dorsal medulla oblongata. The distributions of lesions in the central nervous system of neonatal MSUD are those where the myelination has already occurred at the time of birth, which includes the cerebellar deep white matter, the dorsal brainstem, the cerebral peduncle, the posterior limb of the internal capsule and perirolandic white matter [16, 17]. A previous NS study of neonatal MSUD emphasized the symmetrically increased echogenic lesions located primarily in the periventricular white matter, the basal ganglia and the thalami [17]. These abnormal NS findings were limited in the supratentorial areas, which may not be specific for MSUD. In contrast, the NS characteristics of dorsal brainstem involvement at the level of the pons, which corresponded to the vulnerable myelinated or myelinating regions, is a characteristic sign that may help early diagnosis of this potentially reversible metabolic disease.
Neonatal maple syrup urine disease (gestational age 36 weeks). a The trans-squamous axial NS image at the level of the pons at 8 days old shows hyperechoic lesions (arrow) specifically involving the pontine tegmentum. b The axial T2-weighted MR image (3,500/95) at 19 days old demonstrates the high-signal-intensity abnormalities (arrows) in the same areas.
Birth trauma
Brainstem or spinal cord injury secondary to birth trauma is rarely diagnosed, even after difficult deliveries. Towbin et al. [18], however, found evidence of spinal cord injuries in 10% of newborns during autopsy. It is likely that significant injury to the spinal cord or the lower brainstem during a complicated delivery is more common than is expected. The clinical syndromes of neonatal spinal cord injury include stillbirth or rapid death, severe respiratory failure leading to death and neonatal hypotonia [18]. Injury to the spinal cord and the lower brainstem that occurs during delivery results from excessive longitudinal traction, particularly when combined with flexion of the spinal axis, or rotation of the fetal vertebral column in cephalic as well as breech deliveries. The injury presents a gradient of lesions varying from frank laceration to focal hemorrhage and malacia (Fig. 7), and other parenchymal damage [18]. The diagnosis could often be overlooked because of the accompanying asphyxia and signs of spinal shock. In newborns with acute asphyxia, neurosonography can be the initial neuroimaging modality of choice to evaluate potential traumatic brainstem and spinal cord injury because the newborn need not be moved. The edematous low brainstem or the upper cervical cord may appear echogenic on sagittal trans-foraman magna neurosonography.
Birth trauma in a ventilator-dependent newborn (gestational age 39 weeks). Midsagittal (a) and axial (b) NS imaging through the foramen magnum at 12 days old shows a well-defined ringlike heteroechogenic lesion in the dorsal medulla oblongata (arrow). The midsagittal (c) T2-weighted MR image (4,000/103.1) and the enhanced axial (d) T1-weighted MR image (500/9.8) at 16 days old show a compatible high-signal-intensity lesion extending from the dorsal medulla oblongata to the upper cervical cord, suggesting a subacute hematoma.
Bacterial meningoencephalitis with trans-tentorial temporal lobe herniation
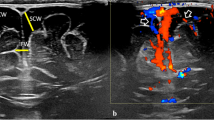
In addition to the alteration of the brainstem parenchymal echogenicity, the change of the contour of the midbrain and its adjacent structures may help in the diagnosis of neonatal devastating neurological disorders as well. The contour of the midbrain in a patient with group B streptococcus meningoencephalitis was found to be distorted by compression at the tegmental areas from the bilateral parahippocampic gyrus, suggesting trans-tentorial temporal lobe herniation (Fig. 8). Herniation of cerebral or cerebellar tissues outside of their supratentoiral or infratentorial compartment secondary to bacterial meningitis is rare in newborns [19]. Two large autopsy reports on young infants with bacterial meningitis specifically remarked on the absence of herniation despite severe brain edema [19]. However, bacterial meningitis complicated with uncal herniations has been neuropathologically documented by Feske et al. [19] in a 5-day-old infant. They also assumed that the rapid rate of development of severe brain edema was critical and containment by the dura rather than the cranium was responsible for the herniation.
Group B streptococcus meningoencephalitis (gestational age 38 weeks). a The trans-squamous axial NS image at 8 days old demonstrates the diffusely heteroechogenic midbrain with a distorted contour which is compressed by herniating bilateral parahippocampi (P). b Unenhanced axial T2-weighted MR imaging (4,000/85.4) 1 month later shows an encephalomalacic change in the midbrain with an irregular contour. The encephalomalacia can also be seen in the other brain parenchyma, including the cerebellum.
Conclusion
Early diagnosis of brainstem involvement requires a high index of clinical suspicion followed by appropriate neuroimaging studies in high-risk newborns. The NS characteristics of brainstem lesions may have diagnostic implications in neonatal neurological disorders. We illustrated that NS imaging through the squamous suture and the foramen magnum can be used as the initial neuroimaging modality in evaluating the brainstem lesions in critically ill neonates with neurological disorders.
References
Huang CC, Chen CY, Yang HB, Wang SM, Chang YC, Liu CC (1998) Central nervous system candidiasis in very low-birth-weight premature neonates and infants: US characteristics and histopathologic and MR imaging correlates in five patients. Radiology 209:49–56
Barkovich AJ (1997) The encephalopathic neonate: choosing the proper imaging technique. Am J Neuroradiol 18:1816–1820
Riccabona M, Nelson TR, Weitzer C, Resch B, Pretorius DP (2003) Potential of three-dimensional ultrasound in neonatal and paediatric neurosonography. Eur Radiol 13:2082–2093
Barr LL (1999) Neonatal cranial ultrasound. Radiol Clin North Am 37:1127–1146
Pigadas A, Thompson JR, Grube GL (1981) Normal infant brain anatomy: correlated real-time sonograms and brain specimens. Am J Roentgenol 137:815–820
Sudakoff GS, Montazemi M, Rifkin MD (1993) The foramen magnum: the underutilized acoustic window to the posterior fossa. J Ultrasound Med 12:205–210
Di Salvo DN (2001) A new view of the neonatal brain: clinical utility of supplemental neurologic US imaging windows. Radiographics 21:943–955
Helmke K, Winkler P, Kock C (1987) Sonographic examination of the brain stem area in infants. An echographic and anatomic analysis. Pediatr Radiol 17:1–6
Yousefzadeh DK, Naidich TP (1985) US anatomy of the posterior fossa in children: correlation with brain sections. Radiology 156:353–361
Khong PL, Lam BC, Tung HK, Wong V, Chan FL, Ooi GC (2003) MRI of neonatal encephalopathy. Clin Radiol 58:833–844
Sugama S, Eto Y (2003) Brainstem lesions in children with perinatal brain injury. Pediatr Neurol 28:212–215
Roland EH, Hill A, Norman MG et al (1988) Selective brainstem injury in an asphyxiated newborn. Ann Neurol 23:89–92
Pasternak JF, Gorey MT (1998) The syndrome of acute near-total intrauterine asphyxia in the term infant. Pediatr Neurol 18:391–398
Sie LT, van der Knaap MS, Oosting J, de Vries LS, Lafeber HN, Valk J (2000) MR patterns of hypoxic–ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics 31:128–136
Kunze K (2002) Metabolic encephalopathies. J Neurol 249:1150–1159
Brismar J, Aqeel A, Brismar G, Coates R, Gascon G, Ozand P (1990) Maple syrup urine disease: findings on CT and MR scans of the brain in 10 infants. Am J Neuroradiol 11:1219–1228
Fariello G, Dionisi-Vici C, Orazi C et al (1996) Cranial ultrasonography in maple syrup urine disease. Am J Neuroradiol 17:311–315
de Vries E, Robben SG, van den Anker JN (1995) Radiologic imaging of severe cervical spinal cord birth trauma. Eur J Pediatr 154:230–232
Feske SK, Carrazana EJ, Kupsky WJ, Volpe JJ (1992) Uncal herniation secondary to bacterial meningitis in a newborn. Pediatr Neurol 8:142–144
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tu, YF., Chen, CY., Lin, YJ. et al. Neonatal neurological disorders involving the brainstem: neurosonographic approaches through the squamous suture and the foramen magnum. Eur Radiol 15, 1927–1933 (2005). https://doi.org/10.1007/s00330-005-2737-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-2737-6