Abstract
Small petrels are the most abundant seabirds in the Southern Ocean. However, because they breed in burrows on remote and often densely vegetated islands, their colony sizes and conservation status remain poorly known. To estimate the abundance of these species on Bird Island in the Falkland archipelago, we systematically surveyed their breeding burrow density and occupancy across this near-pristine tussac (Poa flabellata)-covered island. By modelling burrow density as functions of topography and Sentinel 2 satellite-derived Normalised Difference Vegetation Index data, we inferred habitat associations and predicted burrow abundance of the commonest species—Thin-billed Prions (Pachyptila belcheri) and Wilson’s Storm-petrels (Oceanites oceanicus). We estimate that there are 631,000 Thin-billed Prion burrows on the island (95% CI 496,000–904,000 burrows). Assuming that burrow occupancy lies between 12 and 97%, this equates to around 76,000–612,000 breeding pairs, making Bird Island the second or third largest P. belcheri colony in the world, holding approximately 3–27% of the species’ breeding population. We estimate that 8200–9800 (95% CI 5,200–18,300 pairs) pairs of Wilson’s Storm-petrels also breed on the island. Notably, the latter burrowed predominantly under and within tussac pedestals, whereas they are usually assumed to breed in rock cavities. Thin-billed Prions are declining in the Kerguelen archipelago, but their population trends in the Falklands are unknown. Given the wide confidence intervals around our own and other population estimates for these cryptic species, we recommend that their populations should be monitored regularly, at multiple sites.
Similar content being viewed by others
Introduction
Accurate population estimates are needed to address ecological questions and to inform wildlife management decisions, but many species are difficult to survey directly. In the sub-Antarctic, this problem is exemplified by small- and medium-sized petrels, the most abundant pelagic seabirds of this region. These species breed in burrows, often on remote, densely vegetated islands, making them difficult to survey accurately (Brooke 2004). Uncertainty about the size and trends of petrel populations is of concern because many monitored pelagic seabirds are undergoing rapid declines due to anthropogenic impacts (Croxall et al. 2012; Dias et al. 2019). In many subantarctic regions, including the Falkland Islands, most penguins and albatrosses have been surveyed extensively, but population sizes of cryptic species like burrowing petrels are poorly known. The Falklands comprise ca.750 islands. Few of these have been systematically surveyed for burrowing petrels (Catry et al. 2003; Bolton et al. 2014; Clark et al. 2019), yet many are known or suspected to harbour large proportions of several species’ global populations (Woods 2017).
Bird Island (52°17′ S, 60°93′ W) is a small (1.05 km2) island in the southwest of the Falkland Islands (Fig. 1). It is listed as a National Nature Reserve and Important Bird Area and managed for conservation purposes by the Falkland Islands Government (Falklands Conservation 2006). Although fur seals and penguins were harvested on the island in the 1800s and early 1900s, neither livestock nor other alien plant or animal species were ever introduced (Otley et al. 2008). It is therefore considered to be one of the most pristine tussac (Poa flabellata) covered islands in the Falklands, an archipelago where most islands have been affected by introduced mammalian grazers or predators (Hall et al. 2002; Hilton and Cuthbert 2010). The Falklands archipelago probably holds most of the global population of Thin-billed Prions (Pachyptila belcheri) (Catry et al. 2003), the other main area being the Kerguelen Islands, with smaller numbers on Isla Noir, Chile and 10–20 pairs on the Crozet Islands (Weimerskirch et al. 1989; Marchant and Higgins 1990). During surveys of Striated Caracaras (Phalcoboenus australis) in 1998 and 2006, opportunistic observations indicated that Bird Island holds large populations of burrowing petrels, in particular Thin-billed Prions (Woods 2017). Together with New Island, which holds approximately 2 million breeding pairs, Bird Island is thought to represent the major stronghold of Thin-billed Prions in the Falkland Islands (Catry et al. 2003; Sally Poncet, pers. com). Due to the difficulty of detecting and surveying their colonies, comparatively little is known about the breeding distribution of Wilson’s Storm-petrels (Oceanites oceanicus) in the Falklands (Bolton et al. 2014), but the presence of large aggregations offshore during the breeding season suggests that substantial colonies may exist in the archipelago (Woods and Woods 1997). Prior to our study, Wilson’s Storm-petrels were suspected to breed on Bird Island, but no systematic survey of this or any other burrowing petrels had been undertaken there, leaving considerable uncertainty about their abundance and distribution at this site.
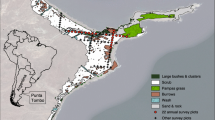
Location of Bird Island (a) and the randomised survey grid (b). NI and SJ are the locations of New Island and Steeple Jason island, respectively. Contours show heights (m) at 10 m intervals and black dots indicate the planned locations of survey plots (note, not all plots were surveyed—see Fig. 2). The extent of tussac was mapped using Google Earth
Conventionally, the number of burrows in seabird colonies are estimated by surveying burrow density using a randomised sampling design, and then multiplying mean burrow density by the extent of the area thought to be occupied (Lawton et al. 2006; Parker and Rexer-Huber 2016). This ‘design-based’ approach is simple but failure to adhere to the randomised design, for example due to logistical constraints, may lead to biazed estimates. In contrast, ‘model-based’ estimation does not require uniform spatial sampling and by using additional habitat information to map burrow density, can potentially lead to more accurate population estimates (Rayner et al. 2007; Clark et al. 2019). However, in order to estimate burrow density in this manner, it is necessary to obtain habitat covariate data covering the entire area of interest. The advent of high-resolution satellite remote sensing has made it much more feasible to collect such data in recent years but to date, remotely sensed habitat data have rarely been used in burrowing petrel studies (Scott et al. 2009). Burrow density models can also be used to make inferences about habitat selection (Olivier and Wotherspoon 2006; Clark et al. 2019). However, because these models do not need to predict across the whole of the study area, it may be preferable to fit them to locally measured covariates, which are usually more accurate than their remotely sensed equivalents. Previous studies suggest that breeding habitat selection by burrowing petrels is non-uniform with respect to topography and ground cover (Beck and Brown 1972; Catry et al. 2003; Olivier and Wotherspoon 2006; Michielsen et al. 2019). Modelling habitat selection by burrowing petrels on Bird Island could therefore provide further insights into the breeding ecology of these species, improve estimates of the distribution and abundance of their burrows and provide important information on the global status of the species.
Our primary aim in this study was to make a rapid but, as far as possible, accurate assessment of the abundance of the most numerous small- and medium-sized petrels breeding on Bird Island, which we found to be Thin-billed Prions and Wilson’s Storm-petrels. In addition, we aimed to quantify breeding habitat selection by these species. To meet these aims, we first systematically surveyed burrow density and occupancy across the island and recorded habitat data. We then modelled burrow density using two sets of models. We used the first set of models, which contained only the locally measured explanatory environmental covariates, to make inferences about habitat selection. We used the second set of models, which contained only remotely sensed explanatory environmental covariates, to predict burrow density, and therefore total burrow abundance across the entire island. We then combined the latter results with burrow occupancy rates obtained during our study and previous studies, in order to estimate the size of petrel breeding populations on the island under different occupancy scenarios. In addition, we report evidence of the presence of other, much less abundant, burrowing petrel species on the island.
Methods
Burrow survey
We conducted fieldwork on Bird Island between 5 and 9th of January, 2018, which coincides with the late incubation/early chick-rearing period of Thin-billed Prions and Wilson’s Storm-petrels in the Falklands (Quillfeldt et al. 2003; Bolton et al. 2014). Other burrowing petrel species that could have been breeding on the island at the time included Sooty Shearwaters (Ardenna grisea), which would have been in their late incubation/early chick-rearing stage (Hedd et al. 2014; Clark et al. 2019); Grey-backed Storm-petrels (Garrodia nereis), which are thought to have an extended breeding period in the Falklands, such that they could have been either incubating or chick-rearing during the study period (Woods and Woods 1997); and Common Diving petrels (Pelecanoides urinatrix). The breeding schedule of the latter in the Falklands is less well known, but on Hummock island (West Falkland) peak hatching occurs in the second half of December (P. Catry, pers. obs.).
To assess the abundance and distribution of the burrows of small- and medium-sized petrels on Bird Island, we used a survey design, adapted from Catry et al. (2003). The survey comprised circular plots of two metre radius (area 12.57 m2) spaced at 75 m intervals on a regular grid placed randomly over the island (Fig. 1). During the survey, four plots proved inaccessible due to steep terrain, so these plots were moved (between 8 and 29 m) to their nearest accessible locations. We aimed to survey as many plots as possible in the time available, whilst surveying a representative range of habitat types. Working in two groups, fieldworkers proceeded northwesterly or southeasterly along one of the rows of plots, surveying each plot as follows. The approximate (± 5 m) location of the plot was found using a GPS. Two fieldworkers then systematically searched for burrows, moving side by side in a circular fashion around the plot, with the outermost fieldworker using a 2 m cord tied to a cane to delimit the outer circumference of the plot. Burrow detection probability was not assessed but we assume, due to the small size of the plots and intensity of search effort, that virtually all burrow entrances were detected. Plots were aligned parallel to the local slope, so burrow counts were per unit surface area, where surface area is defined as the three-dimensional area of the landscape, as opposed to the two-dimensional planar area of the landscape (Parker and Rexer-Huber 2016). Ground cover over most of the island comprised peat, overlain with tussac. Petrel burrows can occur in either substrate (Woods 1970), so we searched both the ground and tussac pedestals. In a small number of plots (2 out of 101) in which scree and boulders also occurred, we checked all rock cavities and recorded these as burrows if they were currently occupied or showed other signs of nesting use (e.g. worn or modified entrances). We recorded burrow abundance by species, determining the species responsible for each burrow from its placement and physical checks of the contents of a subsample of burrows. On tussac-covered islands in the Falklands, Thin-billed Prions and Sooty Shearwaters burrow into the ground, while Common Diving Petrels and Grey-backed Storm-petrels burrow higher in tussac pedestals (Woods 1970). Elsewhere, Wilson’s Storm-petrels typically nest in rock cavities (Marchant and Higgins 1990) but it became apparent during our survey that on Bird Island they also nest in burrows in tussac pedestals (see below). To confirm the species present and obtain an estimate of the proportion of burrows occupied, we checked the contents of the first three burrows found in each plot by hand, removing adults or chicks to determine their species when these were present. Where there was uncertainty over which species occupied other burrows in a plot, these were also checked in this manner. We assumed that burrows that were apparently empty but that were too long or convoluted to check fully were unoccupied, acknowledging that this may result in an underestimate of true occupancy. We recorded the species of any birds or fresh feathers present in the burrow, as well as other signs of occupancy (egg, fresh eggshell or fresh faeces). In addition, for each plot we recorded: the slope, measured ± 5° using a clinometer; the aspect, measured ± 5° using a compass; the percentage ground cover, assessed visually ± 5%; the mean vegetation height, measured ± 5 cm using a graduated pole; and the soil moisture, assessed subjectively on a four point scale from dry to wet following Lawton et al. (2006). Hereafter, we refer to these as locally measured covariates.
Modelling habitat selection
Two burrowing petrel species, Thin-billed Prions and Wilson’s Storm-petrels, were detected actively nesting during the survey (see Results). In order to make inferences about the habitat selection of these species, we modelled burrow counts within survey plots as a function of the locally measured environmental covariates described above using Generalised Additive Models (GAMs), fitted with the mgcv R package (Wood 2017). Hereafter, we refer to these as inference models. GAMs can flexibly fit curved or linear responses to both one-dimensional covariates (e.g. vegetation height) and two-dimensional covariates (e.g. location in space) simultaneously. In our study, this was advantageous firstly because we had little prior expectation of what form associations between burrow density and environmental covariates might take, and secondly, because it allowed us to model the well-known tendency of seabirds to cluster their breeding sites spatially (Coulson 2002; Dilley et al. 2019). To achieve the latter, we included an isotropic smooth of plot coordinates (northings and eastings) in each model (Wood 2017). In addition, to examine interspecific segregation due to competition (Schramm 1986; Ramos et al. 1997; Schumann et al. 2013a), we included the presence of Wilson’s Storm-petrels as a categorical explanatory covariate in the Thin-billed Prion model and vice versa. Prior to model fitting, we transformed aspect using Beer’s transformation (Beers et al. 1966), such that aspect ranged from -1 to 1, where zero is west, the direction of the prevailing wind in the study area. Exploratory analysis indicated that burrow counts were overdispersed, so we assumed that errors followed a negative binomial distribution, checking the validity of this assumption and model fit with q-q and residual plots. We structured smooths of environmental covariates and spatial location as thin-plate regression splines with shrinkage and we set the maximum basis dimension to 4 for the environmental smooths and 15 for the spatial smooth. Model checking following Wood (2017, Sect. 5.9) indicated these dimensions were adequate to avoid over-smoothing. Shrinkage allows model terms to be automatically smoothed to zero during model fitting (indicated by very small effective degrees of freedom), effectively removing the influence of these terms on model predictions. Following Brunbjerg et al. (2018), we calculated the approximate proportion of deviance (d) explained by the ith covariate in each final model, as
where the reduced model does not contain the ith covariate but has the same smoothing parameters as the final model for the remaining smooth covariates. The R script used to fit the models, plus the survey and covariate data, are presented in Online Resources 1 and 2, respectively.
Predicting burrow density and abundance
The locally measured environmental covariates, and the observed density of other species, were only available at each plot location and could not therefore be used to make predictions more widely. In order to predict the density of burrows of Thin-billed Prions and Wilson’s Storm-petrels across the whole island, and thereby their total burrow abundance, for each species, we therefore fitted a similar model to that described above using remotely sensed environmental covariates, which were available for the whole island. Hereafter, we refer to these as prediction models. In order to characterise topography, we downloaded Shuttle Radar Topography Mission, 1 Arc Second Global digital elevation model (DEM) data (NASA 2000) from https://lpdaac.usgs.gov/data_access/data_pool (accessed June 1st, 2017). In the study area, the grid resolution of these data is approximately 30 m. We corrected DEM elevations using ground survey data extracted from a 1:50,000 topographic map (Defence Geographic Centre 2009) and then calculated aspect and slope from the corrected DEM using the raster R package (Hijmans 2019). In order to characterise ground cover, we used a level 1C multi-spectral image captured by the MSI instrument on board the Sentinel 2B satellite, downloaded from the Copernicus Open Access Hub (https://scihub.copernicus.eu/dhus/#/home, accessed November 12th, 2019). We selected the cloud free image recorded most closely in time to the ground survey, which was captured on the December 22nd, 2017 at 13:56 UTC. We then calculated the Normalised Difference Vegetation Index (NDVI):
where RNIR and Rred refer to the reflectance in the near-infrared and red bands, respectively, and band 8 and band 4 refer to the corresponding Sentinel bands. NDVI is a proxy for the amount of photosynthetically active vegetation, and is typically low (~ 0.1–0.2) for bare soil and high (~ 0.3–0.8) for vegetation (Rouse et al. 1974; de Jong et al. 2011). For tussac in the Falkland Islands, it is typically ~ 0.65 (Ilaria Marengo, unpub. data). We resampled the corrected DEM by bilinear interpolation onto the Universal Transverse Mercator grid on which Sentinel band 4 and 8 data were supplied (cell size 10 × 10 m) and carried out all subsequent analyses on this grid.
We specified elevation, aspect, slope, NDVI and location (northings and eastings) as thin-plate regression splines as above and fitted an initial model of burrow density with shrinkage. We then removed terms with low effective degrees of freedom (< 0.3), refitted this model and used it to predict burrow density (burrows m−2 of surface area) in each tussac-covered cell on the 10 × 10 m cell size Universal Transverse Mercator grid described above (burrows of all petrel species were found almost exclusively in tussac-covered area – cf. Figures 1 and 2). To estimate total burrow abundance in each cell, we then multiplied the predicted burrow density in each cell by that cell’s surface area (m2). We estimated the latter from the DEM using the computeAreaRaster function in the mkde R package (Tracey et al. 2014). Finally, we summed the total burrow abundance across all tussac-covered cells on the island. We estimated confidence intervals around this statistic by posterior simulation, using 10,000 draws from the multivariate normal distribution of the model parameters (Wood 2017). Finally, we estimated the breeding population (breeding pairs) by multiplying total burrow abundance by the proportion of burrows occupied.
Observed and predicted density of burrows of Thin-billed Prions (Pachyptila belcheri; left) and Wilson’s Storm-petrels (Oceanites oceanicus; right). Upper panels show means and lower panels their corresponding coefficients of variation (CV). Density was predicted using remotely sensed Copernicus Sentinel multi-spectral data (2019). Only surveyed plots are shown (cf. Figure 1)
Results
During five days of fieldwork on Bird Island, we surveyed 101 of the 168 planned plots (Fig. 2). The majority of plots (88%) were partially or wholly tussac-covered. Bare flat rock occurred in 13% of plots, predominantly near the coast and scree/boulders occurred in only two plots. Petrel burrows occurred almost exclusively in plots that contained tussac (Fig. 2). Thin-billed Prions and Wilson’s Storm-petrel burrows occurred in 66 and 8 surveyed plots, respectively. The two species occurred simultaneously in only one plot and 28 plots contained no burrows. Almost all (97%) of the 951 burrows found belonged to Thin-billed Prions, which occurred at a median density of 0.32 burrows m−2 (range 0.00–3.02 burrows m−2). The remainder (n = 29) were Wilson’s Storm-petrel burrows, all of which occurred in a restricted area in the southwest of the island (Fig. 2), with a median density of 0.07 burrows m−2 (range 0.00–0.56 burrows m−2).
We did not find any burrows unequivocally belonging to other petrel species in the study plots but the following evidence indicated the presence of other species on the island. Firstly, we heard a Sooty Shearwater calling from one burrow (outside the study plots). We also found remains of 4 predated Sooty Shearwaters and saw a raft of up to 300 birds regularly gathering offshore each evening, leading us to believe that a small colony of this species is most likely present. Secondly, we found 4 pairs of Grey-backed Storm-petrel wings in the southwest of the island, along with large numbers of Wilson’s Storm-petrel remains, likely resulting from Short-eared Owl (Asio flammeus) or Brown Skua (Catharacta antarctica) predation. While the remains suggest that Grey-backed Storm-petrels breed on Bird Island, we failed to detect any at night using torches (c.f. Bolton et al. 2014) or to find any burrows in the area where corpses were found. This suggests that they may breed only in small numbers. Finally, we found one desiccated Common Diving Petrel carcass and one Common Diving Petrel was seen flying during 30 min of moon-watching. However, no common diving petrel nests were found and no birds were heard at night. The species is almost certainly scarce on Bird Island, if indeed it breeds there at, but it is important to note that elsewhere in the Falklands burrowing petrel nests are mostly located on cliff edges or along very steep tussac slopes above the sea (Paulo Catry, personal observation). For safety reasons, such areas were not covered during our survey.
Thin-billed Prion burrows occurred almost exclusively in peaty soil, beneath vegetation. Most were in tussac-covered areas and burrow density was higher on the northeast side of the island, especially in elevated areas, and towards the western end of the island (Fig. 2). In contrast, prion burrow density was low or zero in much of the low-lying ground in the middle of the island. The inference model fitted to locally measured covariates for this species explained 69.2% of the deviance in burrow density. Prion burrow density was most strongly associated with vegetation (Table 1), having a humped relationship with vegetation height, peaking at around 90 cm and a positive relationship with percentage vegetation cover (Fig. 3.). The presence of Wilson’s Storm-petrel burrows had next largest effect (Table 1), indicating a negative association between these two species (intercept ± SE = -3.6 ± 1.1, z = -3.27, p = 0.0011). There was also a relatively weak negative association with soil moisture but little evidence of any association with slope or aspect (Fig. 3). Spatial variation not explained by these covariates was relatively small, suggesting a simple increase in burrow density with latitude (Fig. 3). The prediction model for prions (53.7% deviance explained) indicated that burrow density increased with NDVI. Variation in burrow density was predicted largely by this covariate and a relatively complex smooth of location (Fig. 4). Although this model did not contain any topographic explanatory covariates, it is notable that NDVI in survey plots was positively correlated with locally measured vegetation cover (Spearman’s rank correlation ρ = 0.50, p < 0.0001), vegetation height (ρ = 0.31, p = 0.0021) and slope (ρ = 0.30, p = 0.0027). Predicted Thin-billed Prion burrow density was highest on steep slopes on the northeast side of the island, and lowest in the middle of the island (Fig. 2). The estimated surface area of tussac-covered cells on the prediction grid ranged from 100 to 220 m2 (median 103 m2). In total, the model fitted to the remotely sensed data predicts that there were 630,517 (95% CI 500,460–903,536; Table 1) Thin-billed Prion burrows on the island at the time of the survey.
Partial response to locally measured explanatory covariates included in the inference models of burrows density (burrows m−2) for Thin-billed Prions (Pachyptila belcheri; left) and Wilson’s Storm-petrels (Oceanites oceanicus; right). The Generalised Additive Models assume a negative binomial error distribution, with a log link function and their intercepts are -1.60 (SE ± 0.20) and -14.5 (SE ± 5.38), respectively. In addition, each model contained the presence of other species as a categorical explanatory covariate (see text for details). P-values indicate the approximate significance of each smooth term and the number in each y-axis label indicates the effective degrees of freedom used by that smooth. The bottom panels show the response to the spatial coordinates of the survey plots. In the upper panels, shaded regions show approximate 95% CIs and tick marks show the observations. In the lower panels, dashed lines indicate + (green/dotted) or – (red/dashed) one standard error about the mean contours (black) and black dots show the locations of the survey plots
Partial response to remotely sensed Normalised Difference Vegetation Index (NDVI—upper panels) and location (lower panels) in prediction models of burrows density (burrows m−2) for Thin-billed Prions (Pachyptila belcheri; left) and Wilson’s Storm-petrels (Oceanites oceanicus; right). The Generalised Additive Models assume a negative binomial error distribution, with a log link function. Their intercepts are = 1.43 (SE ± 0.13) and -6.15 (SE ± 0.72), respectively. Annotation is as in Fig. 3
We found Wilson’s Storm-petrel burrows only in an area of dense tussac on the southwest end of the island (Fig. 2). Burrows were located in both tussac pedestals and the soil beneath. The inference model (fitted to locally measured covariates) explained 74.2% of the deviance in burrow density. The largest amount of deviance was explained by the spatial smooth of plot location (Fig. 3, Table 1). The model also indicated a negative association with Thin-billed Prions (intercept ± SE = − 2.5 ± 0.8, z = − 3.04, p = 0.0021) and a positive association with vegetation height (Fig. 3). The most parsimonious remotely sensed prediction model (57.2% deviance explained) indicated that Wilson’s Storm-petrel burrow density was best predicted by location and a positive relationship with NDVI (Table 1). However, the slope of the former relationship was relatively uncertain (Fig. 4). As a result, the confidence interval around the total predicted number of Wilson’s Storm-petrel burrows (14,209) was wide (95% CI 8,974–26,494 burrows; Table 2).
Based on the confirmed presence of an adult, egg or chick, a minimum of 12% (95% CI: 8–18%, n = 178) of Thin-billed Prion and ~ 58% (95% CI 35–78%, n = 19) of Wilson’s Storm-petrel burrows were definitely occupied. If presence of fresh guano or feathers in the burrow is also considered as evidence of occupation, the equivalent figure was 58% (95% CI 51–66%) for prions, while that of Wilson’s Storm-petrels is unchanged. All but one occupied putative Wilson’s Storm-petrel burrow (i.e. 10 out of 19) contained an incubating adult, making it possible to discriminate these burrows unambiguously from those of Grey-backed Storm-petrels (the remaining burrow contained an egg). Assuming that Thin-billed Prion occupancy on Bird Island lies between our minimum estimate (12%) and the maximum observed elsewhere (97%, Catry et al. 2003), the total Thin-billed Prion breeding population on Bird Island would be between 75,662 (95% CI 59,501–108,479) and 611,601 (95% CI 480,970–876,873) pairs (Table 2). Equivalent values for Wilson’s Storm-petrels are 8241 (95% CI 5205–15,367) to 9804 (95% CI 6192–18,281) pairs. Breeding population estimates based on other plausible occupancy rates are detailed in Table 2.
Discussion
Our results show that Bird Island is one of the largest known single-island colonies of Thin-billed Prions in the world. Further, they provide the first formal size estimate for a Wilson’s Storm-petrel colony in the Falkland Islands, flagging Bird Island as a regionally important site for this species. In addition, our models provide insights into breeding site selection, in particular by Thin-billed Prions. We discuss these results in more detail, including their potential limitations and wider significance.
The habitat use models fitted to locally measured environmental covariates indicated that for both Thin-billed Prions and Wilson’s Storm-petrels, the negative association with one another had one of the largest effects of all covariates (Fig. 2, Table 1). This could arise due to competitive exclusion—for example, if Thin-billed Prions excluded smaller Wilson’s Storm-petrels from areas containing habitat that would be suitable for either species (Schramm 1986; Ramos et al. 1997; Schumann et al. 2013a). Alternatively, the two species could have differing habitat preferences (Woods 1970; Schumann et al. 2013a). Although both species nested in dense tussac on Bird Island, we noticed in the field that the tussac in the Wilson’s Storm-petrel dominated area was extremely dense, with almost no bare soil between adjacent pedestals. Another (not necessarily mutually exclusive) possibility is that each species preferentially nests near conspecifics for social reasons, with isolated monospecific clusters arising due to social attraction (Melles et al. 2009). Ultimately, further study would be required to disentangle these effects. This could be important because the tendency for seabirds to cluster due to social effects gives rise to otherwise unexplained spatial autocorrelation in habitat models, and is one reason why habitat use models fitted to data from one location may predict poorly in others (Clark et al. 2019). In addition, although the general assumption is that Wilson’s Storm-petrels tend to breed only in rock cavities (Weimerskirch et al. 1989; Brooke 2004; Quillfeldt et al. 2005; Olivier and Wotherspoon 2006), we found them mostly in areas with dense (and sometimes very tall) tussac, completely devoid of exposed rock. Moreover, most burrows were constructed in the tussac vegetation, rather than in the ground and were therefore very similar to Grey-backed Storm-petrels (Woods 1970). Although not unprecedented (Marchant and Higgins 1990), this suggests that large colonies of Wilson’s Storm-petrels could have gone undetected in other subantarctic areas and that their regional and global population sizes may therefore have been underestimated. Indeed, although almost all of the occupied storm-petrel burrows that we checked contained an adult Wilson’s Storm-petrel and could therefore be unambiguously assigned to this species (one burrow contained only an egg), it is possible that some of the unoccupied burrows belonged to Grey-backed Storm-petrels. If so, this would have led us to overestimate the total abundance of Wilson’s Storm-petrel burrows but not their breeding population size, because the latter is corrected for occupancy.
Our results indicate that Thin-billed Prion breeding habitat associations on Bird Island are somewhat different to those on nearby islands. For example, whereas the density of Thin-billed Prions on Bird Island was low in wet areas and those with sparse vegetation, and highest in areas where the mean vegetation height was around 90 cm, burrow density on nearby New Island was highest in areas with sparse vegetation, particularly those covered with short grasses and the introduced sheep sorrel (Rumex acetosella) (Catry et al. 2003). In addition, while Thin-billed Prion burrow density on New Island and Steeple Jason (also in the western Falklands) tends to be higher on steeper slopes (Catry et al. 2003; Bolton et al. 2014), none of our models indicated a positive association between prion burrow density and slope on Bird Island. This could indicate that the drivers of burrow site selection are complex and differ markedly from location to location. For example, on Mayes Island in the Kerguelen Archipelago, Thin-billed Prions burrow mainly in stony areas with relatively little vegetation cover (Genevois and Buffard 1994). Although this is possibly due to competition from sympatric blue petrels (Halobaena caerulea), it further illustrates that breeding habitat selection is relatively plastic among Thin-billed Prions. Notwithstanding this flexibility, if Thin-billed Prions on Bird Island have similar topographic preferences to those on nearby New Island and Steeple Jason and vegetation cover and height vary systematically with slope, it would suggest that we were able to measure slope less accurately than vegetation. This could certainly be the case in the prediction model, in which slope was calculated using the only available DEM data, which had a spatial resolution of 30 m. In contrast, NDVI—our proxy for vegetation—had a spatial resolution of 10 m. Ultimately, surveys using standard methods across multiple islands with differing ground cover would be required to test these hypotheses.
In our study, we were able to predict burrow density across Bird Island relatively well using just NDVI and spatial location. The former is likely to be an effective predictor because it is correlated with vegetation height and vegetation cover measured at the plot level. Although habitat modelling is used relatively infrequently to estimate burrowing petrel populations (Rayner et al. 2007; Scott et al. 2009; Clark et al. 2019), its judicious use could provide improvements in efficiency and accuracy over traditional design-based estimation. An important caveat, however, is that extrapolation into unsampled geographical or environmental space is likely to give poor predictions, not least because habitat associations can vary markedly from location to location (Scott et al. 2009; Whitehead et al. 2014; Clark et al. 2019), for example due to differences in habitat availability among those locations (Matthiopoulos et al. 2011). One advantage of model-based estimation is that it allows deviation from entirely randomised survey designs. This was very useful in our case, because limited time on the island meant that we had to deviate from the planned randomised survey design which would have biazed the sampling if these deviations were correlated with habitat type or bird abundance in some way. For example, many of the plots missed in our study were in the middle of the island, where burrow density was low. A purely design-based estimate based on our sample would therefore overestimate total burrow abundance but by modelling the relationship between burrow density and habitat, we avoided this pitfall. A further problem common to both model-based and design-based estimation is that burrowing petrels often breed in locations too dangerous or impractical to survey (e.g. Granadeiro et al. 2006; Lavers 2014). In our case, this may have led to an underestimate of the number of Thin-billed Prion burrows, and possibly smaller clusters of other species, because we could not survey the very steep vegetated cliffs on the north and southeast sides of Bird Island. In addition, leaving the steepest habitats un-surveyed may have resulted in our failure to confirm nesting common diving petrels on the island. We also saw a Wilson’s Storm-petrel in low flight over a small boulder beach, a habitat type not captured in our field survey, where it is possible that some nests of this species went undetected.
In order to estimate the abundance of burrowing petrels, it is necessary estimate not only the number of burrows but the proportion occupied. For logistical reasons, it was impracticable for us to visit Bird Island until early January so we could not measure peak burrow occupancy, which tends to occur just after laying has finished, declining subsequently due to breeding failures (Parker and Rexer-Huber 2016). Moreover, we inspected burrows by hand, which is unlikely to result in perfect detection of adults/eggs/chicks (Schumann et al. 2013b; Parker and Rexer-Huber 2016). As such, our lower prion occupancy estimate (12%) should be regarded as a minimum. Including the presence of fresh guano or feathers in burrows as a further signs of occupancy resulted in a much higher estimate (58%). However, the latter could be left by prospecting non-breeders, rather than breeding adults resulting in false positives that would bias apparent occupancy upward (Clark et al. 2019). Despite these caveats, the Thin-billed Prion occupancy rates that we observed were within the bounds reported in previous studies in the Falklands and Kerguelen, which range widely, from 10 to 95% (Catry et al. 2003; Quillfeldt et al. 2003; Pacoureau et al. 2019). Interestingly, our occupancy estimates for Wilson’s Storm-petrels were the same whether occupancy was defined by the presence of an adult/egg/chick or additionally by the presence of feathers/guano. We suspect that this is because despite the small diameter of these burrows, they were constructed within relatively flexible substrates, allowing them to be searched thoroughly by hand. The occupancy rate that we observed for Wilson’s Storm-petrels (58%) is near the high end of the range reported for other sites (9 to 69%) but these estimates all come from Antarctica, where the species breeds in rock cavities, which are more difficult to search (Beck and Brown 1972; Quillfeldt 2004; Barbraud et al. 2018). We are unaware of any other studies on the occupancy rates of Wilson’s Storm-petrels in tussac habitats or in the subantarctic.
It has been estimated that the world population of Thin-billed Prions is around 7 million individuals, equivalent to ~ 2.3 million breeding pairs, while that of Wilson’s Storm-petrels is ~ 4–10 million breeding pairs (Brooke 2004). Assuming that true occupancy on Bird Island lies between our minimum estimates and the maxima observed elsewhere, around 3–27 and 0.1–0.3% of the world populations of these species, respectively, breed on Bird Island (Table 2). However, we caution that these are very approximate estimates, because in addition to the sources of uncertainty about occupancy discussed above, one burrow entrance can lead to multiple nest chambers (Catry et al. 2003; Pacoureau et al. 2019), and detection of burrows may be imperfect (Barbraud et al. 2018), either of which could have resulted in an underestimation of burrow abundance in our study. In particular, uncertainty around our estimate of the number of Wilson’s Storm-petrel burrows was large because this species was present in only 8 out 101 survey plots, all located in the southwest of the island. Future surveys could refine our population estimate for this species by surveying this area more intensively.
The results from the present survey indicate that, even at the lower end of our burrow estimates, Bird Island harbours the second or third largest known Thin-billed Prion colony in the world, after New Island in the Falklands, and possibly after Kerguelen mainland (Weimerskirch et al. 1989 and pers. com). Although the species is present at many other sites in the Falklands, no other islands are suspected to harbour colonies of comparable size to New or Bird Islands (Sally Poncet, pers. com.). Thin-billed Prions are also abundant on various other islands of the Kerguelen archipelago, but probably in much smaller numbers than on Bird Island (Henri Weimerskirch & Christophe Barbraud, pers. com.). The situation in Chile has never been clarified, although a moderately large colony is likely to be present on Isla Noir (Brooke 2004 and Alejandro Kusch, pers com). Overall, population estimates are either relatively crude guesses, or more formal estimates with large confidence intervals, as in the present study. The challenges of obtaining accurate estimates of both density and burrow occupancy in burrowing petrel populations worldwide seem to be massive. Furthermore, most nesting colonies, including that on Bird Island, are remote and logistically challenging to survey. As such, population estimates are likely to remain crude, but field surveys like ours remain a priority, if we are to identify sites of greatest conservation value. Evaluating trends in populations of these cryptic species will probably require establishing protocols for long-term monitoring, and subsequently conducting surveys in small, representative plots, where trends can be assessed with greater accuracy. Large-scale surveys for population monitoring remain challenging and largely impractical, and even the use of remote sensing and advanced modelling, like in our study, suffer from major limitations in their ability to produce estimates with narrow confidence intervals that could potentially be used to assess population trends.
Thin-billed Prion populations are apparently declining rapidly in Kerguelen (Pacoureau et al. 2019), which may be due to climate-driven oceanographic changes, as predicted by Nevoux & Barbraud (2006). Furthermore, the main Thin-billed Prion colony in this region (on the Rallier du Baty Peninsula) is threatened by invasion by feral cats, which would likely result in local collapse or extinction, as has occurred elsewhere on mainland Kerguelen (Henri Weimerskirch, pers. com.). Of critical concern, the main Falkland colony, on New Island, also suffers from predation by introduced cats and rats (Catry et al. 2007). Nothing is known of the present population trend of Thin-billed Prions in the Falklands. However, islands in the archipelago, such as Bird Island, likely represent their main stronghold worldwide. Hence, increased frequency of monitoring of this species, ideally at multiple sites with differing habitat characteristics, including Bird Island, is highly desirable and an urgent priority. Moreover, Bird Island deserves careful ongoing management to protect its near-pristine state and high biodiversity.
References
Barbraud C, Vasseur J, Delord K (2018) Using distance sampling and occupancy rate to estimate abundance of breeding pairs of Wilson’s storm petrel (Oceanites oceanicus) in Antarctica. Polar Biol 41:313–322. https://doi.org/10.1007/s00300-017-2192-2
Beck JR, Brown DW (1972) The biology of Wilson’s storm petrel, Oceanites oceanicus (Kuhl), at Signy Island, South Orkney Islands. Scientific Rep Br Antarct Surv No 69:1–54
Beers TW, Dress PE, Wensel LC (1966) Aspect transformation in site productivity research. J For 64:691–692
Bolton M, Stanbury A, Baylis AMM, Cuthbert R (2014) Impact of introduced house mice (Mus musculus) on burrowing seabirds on steeple jason and Grand Jason Islands, Falklands, South Atlantic. Polar Biol 37:1659–1668. https://doi.org/10.1007/s00300-014-1554-2
Brooke M (2004) Albatrosses and Petrels Across the World. Oxford University Press, Oxford
Brunbjerg AK, Hale JD, Bates AJ, Fowler RE, Rosenfeld EJ, Sadler JP (2018) Can patterns of urban biodiversity be predicted using simple measures of green infrastructure? Urban Forestry & Urban Greening 32:143–153. https://doi.org/10.1016/j.ufug.2018.03.015
Catry P, Campos A, Segurado P, Silva M, Strange I (2003) Population census and nesting habitat selection of thin-billed prion Pachyptila belcheri on New Island, Falkland Islands. Polar Biol 26:202–207. https://doi.org/10.1007/s00300-002-0465-9
Catry P, Silva MC, MacKay S, Campos A, Masello J, Quillfeldt P, Strange IJ (2007) Can thin-billed prions Pachyptila belcheri breed successfully on an island with introduced rats, mice and cats? The case of New Island, Falkland Islands. Polar Biol 30:391–394. https://doi.org/10.1007/s00300-006-0204-8
Clark TJ, Matthiopoulos J, Bonnet-Lebrun A-S, Campioni L, Catry P, Marengo I, Poncet S, Wakefield E (2019) Integrating habitat and partial survey data to estimate the regional population of a globally declining seabird species, the sooty shearwater. Global Ecol Conserv 17:e00554. https://doi.org/10.1016/j.gecco.2019.e00554
Conservation F (2006) Falkland Islands Important Bird Areas: Bird Island. Falklands Conservation, Stanley, Falkland Islands
Coulson JC (2002) Colonial breeding in seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, Boca Raton, pp 87–113
Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan BJ, Symes A, Taylor P (2012) Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv Inte 22:1–34
de Jong R, de Bruin S, de Wit A, Schaepman ME, Dent DL (2011) Analysis of monotonic greening and browning trends from global NDVI time-series. Remote Sens Environ 115:692–702. https://doi.org/10.1016/j.rse.2010.10.011
Defence Geographic Centre (2009) Series H792. Defence Geographic Centre, ICG, Ministry of Defence, United Kingdom, Crown Copyright, p 2009
Dias MP, Martin R, Pearmain EJ, Burfield IJ, Small C, Phillips RA, Yates O, Lascelles B, Borboroglu PG, Croxall JP (2019) Threats to seabirds: a global assessment. Biol Conserv 237:525–537. https://doi.org/10.1016/j.biocon.2019.06.033
Dilley BJ, Hedding DW, Henry DAW, Rexer-Huber K, Parker GC, Schoombie S, Osborne A, Ryan PG (2019) Clustered or dispersed: testing the effect of sampling strategy to census burrow-nesting petrels with varied distributions at sub-Antarctic Marion Island. Antarctic Science 31:231–242. https://doi.org/10.1017/S0954102019000300
Genevois F, Buffard E (1994) Nesting sites and burrow characteristics of two sympatric species on the Kerguelen Islands (Antarctic): The blue petrel Halobaena caerulea and the thin-billed prion Pachyptila belcheri. Alauda 62:123–134
Granadeiro JP, Dias MP, Rebelo R, Santos CD, Cartry P (2006) Numbers and population trends of Cory’s shearwaters Calonectris diomedea at Selvagem Grande, Northeast Atlantic. Waterbirds 29:56–60
Hall JR, Woodsde RW, Brooke L, Hilton M (2002) Factors affecting the distribution of landbirds on the Falkland Islands. Bird Conservation International 12:151–167. https://doi.org/10.1017/S0959270902002095
Hedd A, Montevecchi WA, Phillips RA, Fifield DA (2014) Seasonal sexual segregation by monomorphic sooty shearwaters Puffinus griseus reflects different reproductive roles during the pre-laying period. PLoS ONE 9:e85572. https://doi.org/10.1371/journal.pone.0085572
Hijmans RJ (2019) raster: Geographic Data Analysis and Modeling. R package version 2.9–5. https://CRAN.R-project.org/package=raster.
Hilton GM, Cuthbert RJ (2010) Review article: The catastrophic impact of invasive mammalian predators on birds of the UK Overseas Territories: a review and synthesis. Ibis 152:443–458. https://doi.org/10.1111/j.1474-919X.2010.01031.x
Lavers JL (2014) Population status and threats to flesh-footed Shearwaters (Puffinus carneipes) in South and Western Australia. ICES J Mar Sci 72:316–327. https://doi.org/10.1093/icesjms/fsu164
Lawton K, Robertson G, Kirkwood R, Valencia J, Schlatter R, Smith D (2006) An estimate of population sizes of burrowing seabirds at the Diego Ramirez archipelago, Chile, using distance sampling and burrow-scoping. Polar Biol 29:229–238. https://doi.org/10.1007/s00300-005-0043-z
Marchant S, Higgins PJ (1990) Handbook of Australian, New Zealand & Antarctic Birds, vol 1. Oxford University Press, Melbourne
Matthiopoulos J, Hebblewhite M, Aarts G, Fieberg J (2011) Generalized functional responses for species distributions. Ecology 92:583–589. https://doi.org/10.1890/10-0751.1
Melles SJ, Badzinski D, Fortin MJ, Csillag F, Lindsay K (2009) Disentangling habitat and social drivers of nesting patterns in songbirds. Landsc Ecol 24:519. https://doi.org/10.1007/s10980-009-9329-9
Michielsen RJ, Ausems ANMA, Jakubas D, Pętlicki M, Plenzler J, Shamoun-Baranes J, Wojczulanis-Jakubas K (2019) Nest characteristics determine nest microclimate and affect breeding output in an Antarctic seabird, the Wilson’s storm-petrel. PLoS ONE 14:e0217708. https://doi.org/10.1371/journal.pone.0217708
NASA (2000) Shuttle Radar Topography Mission, 1 Arc Second Global. NASA EOSDIS Land Processes DAAC, USGS Earth Resources Observation and Science (EROS) Center, Sioux Falls, South Dakota
Nevoux M, Barbraud C (2006) Relationships between sea ice concentration, sea surface temperature and demographic traits of thin-billed prions. Polar Biol 29:445–453. https://doi.org/10.1007/s00300-005-0075-4
Olivier F, Wotherspoon SJ (2006) Distribution and abundance of Wilson’s storm petrels Oceanites oceanicus at two locations in East Antarctica: testing habitat selection models. Polar Biol 29:878–892. https://doi.org/10.1007/s00300-006-0127-4
Otley H, Munro G, Clausen A, Ingham B (2008) Falkland Islands State of the Environment Report 2008. Falkland Islands Government and Falklands Conservation, Stanley
Pacoureau N, Gaget E, Delord K, Barbraud C (2019) Prey remains of brown skua is evidence of the long-term decline in burrow occupancy of blue petrels and thin-billed prions at Mayes Island, Kerguelen. Polar Biol 42:1873–1879. https://doi.org/10.1007/s00300-019-02567-4
Parker GC, Rexer-Huber K (2016) Guidelines for designing burrowing petrel surveys to improve population estimate precision. Agreement on the Conservation of Albatrosses and Petrels http://www.acap.aq/en/resources/acap-conservation-guidelines. Accessed 7 August 2017,
Quillfeldt P (2004) Variation in breeding success in Wilson’s storm petrels: influence of environmental factors. Antarct Sci 13:400–409. https://doi.org/10.1017/S0954102001000566
Quillfeldt P, Masello JF, Strange IJ (2003) Breeding biology of the thin-billed prion Pachyptila belcheri at New Island, Falkland Islands: egg desertion, breeding success and chick provisioning in the poor season 2002/2003. Polar Biol 26:746–752. https://doi.org/10.1007/s00300-003-0544-6
Quillfeldt P, McGill RAR, Furness RW (2005) Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson’s storm-petrel. Mar Ecol Prog Ser 295:295–304. https://doi.org/10.3354/meps295295
Ramos JA, Monteiro LR, Sola E, Moniz Z (1997) Characteristics and competition for nest cavities in burrowing procellariiformes. The Condor 99:634–641. https://doi.org/10.2307/1370475
Rayner MJ, Clout MN, Stamp RK, Imber MJ, Brunton DH, Hauber ME (2007) Predictive habitat modelling for the population census of a burrowing seabird: a study of the endangered cook’s petrel. Biol Conserv 138:235–247. https://doi.org/10.1016/j.biocon.2007.04.021
Rouse JW, Haas RH, Schell JA, Deering DW (1974) Monitoring vegetation systems in the Great Plains with ERTS. Paper presented at the Proceedings of the Third Earth Resources Technology Satellite- 1 Symposium, Greenbelt, NASA SP-351,
Schramm M (1986) Burrow densities and nest site preferences of petrels (procellariidae) at the prince Edwards Islands. Polar Biol 6:63–70. https://doi.org/10.1007/BF00258254
Schumann N, Dann P, Arnould JPY (2013a) Use of terrestrial habitats by burrow-nesting seabirds in south-eastern Australia. Emu 113:135–144. https://doi.org/10.1071/MU12088
Schumann N, Dann P, Hoskins AJ, Arnould JPY (2013b) Optimizing survey effort for burrow-nesting seabirds. J Field Ornithol 84:69–85. https://doi.org/10.1111/jofo.12007
Scott D, Moller H, Fletcher D, Newman J, Aryal J, Bragg C, Carleton K (2009) Predictive habitat modelling to estimate petrel breeding colony sizes: sooty shearwaters (Puffinus griseus) and mottled petrels (Pterodroma inexpectata) on Whenua Hou Island. New Zealand J Zool 36:291–306
Tracey JA, Sheppard J, Zhu J, Sinkovts R, Chourasia A, Lockwood G, Fisher RN (2014) mkde: 2D and 3D movement-based kernel density estimates (MKDEs). R package version 0.1. https://CRAN.R-project.org/package=mkde.
Weimerskirch H, Zotier R, Jouventin P (1989) The Avifauna of the Kerguelen Islands. Emu 89:15–29. https://doi.org/10.1071/MU9890015
Whitehead A, Lyver P, Jones C, Bellingham P, MacLeod C, Coleman M, Karl B, Drew K, Pairman D, Gormley A, Duncan R (2014) Establishing accurate baseline estimates of breeding populations of a burrowing seabird, the grey-faced petrel (Pterodroma macroptera gouldi) in New Zealand. Biol Conserv 169:106–116. https://doi.org/10.1016/j.biocon.2013.11.002
Wood SN (2017) Generalized Additive Models: An Introduction with R, 2nd edn. Chapman and Hall/CRC Boca Raton, Florida
Woods RW (1970) The avian ecology of a tussock island in the Falkland Islands. Ibis 112:15–24. https://doi.org/10.1111/j.1474-919X.1970.tb00072.x
Woods RW (2017) The Birds of the Falkland Islands An Annotated Checklist. British Ornithologists’ Club, Tring, UK
Woods RW, Woods A (1997) Atlas of breeding birds of the Falkland Islands. Anthony Nelson, Oswestry, UK
Acknowledgements
Funding was provided by the Falkland Islands Government Environmental Studies Budget; Fundação para a Ciência e a Tecnologia, Portugal (Grants UIDB/04,292/2020 and UIDP/04,292/2020 to MARE) and the UK Natural Environment Research Council (Grant NE/M017990/1 to EW). Logistical support was generously provided by the South Atlantic Environmental Research Institute. We are grateful to Jérôme Poncet for providing transport to the field site and to the staff of Weddell Island for logistical support. Mark Bolton, Kalinka Rexer-Huber and an anonymous reviewer provided helpful criticism on a previous version of this paper.
Funding
Falkland Islands Government Environmental Studies Budget; Fundação para a Ciência e a Tecnologia, Portugal (Grants UIDB/04292/2020 and UIDP/04292/2020 to MARE) and the UK Natural Environment Research Council (Grant NE/M017990/1).
Author information
Authors and Affiliations
Contributions
EW, PC and JM designed and secured funding for the study. All authors except JM collected the data, which AS, EW and JM analysed. AS, EW and PC wrote the manuscript, which all authors then edited.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
300_2021_2842_MOESM1_ESM.csv
Supplementary file1 CSV file containing survey and covariate data. Includes one row for each cell on the prediction grid (CSV 1475 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stokes, A.W., Catry, P., Matthiopoulos, J. et al. Combining survey and remotely sensed environmental data to estimate the habitat associations, abundance and distribution of breeding thin-billed prions Pachyptila belcheri and Wilson’s storm-petrels Oceanites oceanicus on a South Atlantic tussac island. Polar Biol 44, 809–821 (2021). https://doi.org/10.1007/s00300-021-02842-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02842-3