Abstract
Although the Arctic cod (Boreogadus saida) has a pan-Arctic distribution, little is known about its occurrence in near-shore waters where this species is the principal prey for seabirds, marine mammals and other fish. Published research describes the scyphomedusa Cyanea capillata as an Arctic cod predator, and this paper presents observations from long-term investigations using active hydroacoustics that suggest the Arctic cod avoided C. capillata in two small bays of Cornwallis Island (Canadian High Arctic archipelago). Distribution patterns in echograms suggested that features such as boundary layer fronts restricted jellyfish movements and Arctic cod were often abundant on the side of fronts where C. capillata were absent. Thus, habitat partitioning allowed Arctic cod to share habitat with its predator, albeit exceptions to this sharing occurred when jellyfish abundance was high and Arctic cod were displaced. Thus, if a warmer Arctic triggers an increase in C. capillata abundance, it is possible that small-scale aspects of Arctic cod distribution could be affected. This in turn could have significant ripple effects within the Arctic food web, an additional and previously unrecognized consequence of climate change.






Similar content being viewed by others
References
Arai MN (1988) Interactions of fish and pelagic coelenterates. Can J Zool 66:1913–1927
Arai MN (2001) Pelagic coelenterates and eutrophication: a review. Hydrobiologia 451:69–87
Baxter EJ, Rodger HD, McAllen R (2011) Gill disorders in marine-farmed salmon: investigating the role of hydrozoan jellyfish. Aquacult Environ Interact 1:245–257. doi:10.3354/aei00024
Benoit D, Simard Y, Fortier L (2008) Hydroacoustic detection of large winter aggregations of Arctic cod (Boreogadus saida) at depth in ice-covered Franklin Bay (Beaufort Sea). J Geophys Res 113:C06S90. doi:10.1029/2007JC004276
Bradstreet SW, Cross WE (1982) Trophic relationships of high ice edges. Arctic 35:1–12
Bradstreet MSW, Finley KJ, Sekerak AD, Griffiths WB, Evans CR, Fabijan MF, Stallard HE (1986) Aspects of the biology of arctic cod (Boreogadus saida) and its importance in arctic marine food chains. Can Tech Rep Fish Aquat Sci 1491:193
Brierley AS, Axelsen BE, Boyer DC, Lynam CP, Didcock CA, Boyer HJ, Sparks CAJ, Purcell JE, Gibbons MJ (2004) Single-target echo detections of jellyfish. ICES J Mar Sci 61:383–393
Brodeur RD, Mills CE, Overland JE, Walters GE, Schumacher JD (1999) Evidence for a substantial increase in gelatinous zooplankton in the Bering Sea, with possible links to climate change. Fish Oceanogr 8:296–306
Brodeur RD, Sugisaki H, Hunt GL Jr (2002) Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Mar Ecol Prog Ser 233:89–103
Crawford RE (1988) Fisheries acoustics in the High Arctic: under-ice applications, pp 95–98. In: Department of Fisheries and Oceans 1988 Hydroacoustic workshop proceedings. Can Tech Rep Fish Aquat Sci 1641, p 111
Crawford RE, Carey CG (1985) Retention of winter flounder larvae within a Rhode Island salt pond. Estuaries 8:217–227
Crawford RE, Fox JK (1992) Visualization of echo sounder data with a micro computer. Can Tech Rep Fish Aquat Sci. 1840:17
Crawford R, Jorgenson J (1990) Density distribution of fish in the presence of whales at the Admiralty Inlet landfast ice edge. Arctic 43:215–222
Crawford RE, Jorgenson J (1993) Schooling behaviour of Arctic cod in relation to drifting pack ice. Envir Biol Fish 36:345–357. doi:10.1007/BF00012412
Crawford RE, Jorgenson J (1996) Quantitative studies of Arctic cod (Boreogadus saida) schools: important energy stores in the Arctic food web. Arctic 49:181–193
Crawford RE, Vagle S, Carmack EC (2012) Water mass and bathymetric characteristics of Arctic cod habitat along the continental shelf and slope of the Beaufort and Chukchi seas. Polar Biol 35:179–190. doi:10.1007/s00300-011-1051-9
Graham WM, Pagés F, Hamner WM (2001) A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia 451:199–212
Greene JM (1983) Observations on the food of marine fishes from Resolute Bay, Cornwallis Island, Northwest Territories. Astarte J Arctic Biol 12:63–68
Harter BB, Elliott KH, Divoky GL, Davoren GK (2013) Arctic cod (Boreogadus saida) as prey: fish length-energetics relationships in the Beaufort Sea and Hudson Bay. Arctic 66:191–196
Hop H, Welch H, Crawford R (1997) Population structure and feeding ecology of Arctic cod schools in the Canadian High Arctic. In: Reynolds J (ed) Fish ecology in Arctic North America. American Fisheries Society Symposium 19, Bethesda, Maryland, pp 68–78
Hudon C, Crawford RE, Ingram RG (1993) Influence of physical forcing on the spatial distribution of marine fauna near Resolution Island (eastern Hudson Strait). Mar Ecol Prog Ser 92:1–14
Kessel ST, Hussey NE, Crawford RE, O’Neill CV, Yurkowski DJ, Fisk AT (2015) Distinct patterns of Arctic cod (Boreogadus saida) presence and absence in a shallow high Arctic embayment, revealed across open water and ice covered periods through acoustic telemetry. Polar Biol. doi:10.1007/s00300-015-1723-y
Lønne OJ, Gulliksen B (1989) Size, age, and diet of polar cod, Boreogadus saida (Lepechin 1773), in ice covered waters. Polar Biol 9:187–191
Lowry LF, Frost KJ (1981) Distribution, growth and foods of Arctic cod (Boreogadus saida) in the Bering, Chukchi and Beaufort seas. Can Field Nat 95:186–191
Lynam CP, Heath MR, Hay SJ, Brierley AS (2005) Evidence for impacts by jellyfish on North Sea herring recruitment. Mar Ecol Prog Ser 298:157–167
Majaneva S, Berge J, Renaud PE, Vader A, Stübner E, Rao AM, Sparre Ø, Lehtiniemi M (2013) Aggregations of predators and prey affect predation impact of the Arctic ctenophore Mertensia ovum. Mar Ecol Prog Ser 476:87–100. doi:10.3354/meps10143
Matley JK, Crawford RE, Dick TA (2012a) Summer foraging behaviour of shallow-diving seabirds and distribution of their prey, Arctic cod (Boreogadus saida), in the Canadian Arctic. Polar Res. doi:10.3402/polar.v31i0.15894
Matley JK, Crawford RE, Dick TA (2012b) Observation of common raven (Corvus corax) scavenging Arctic cod (Boreogadus saida) from seabirds in the Canadian High Arctic. Polar Biol 35:1119–1122. doi:10.1007/s00300-011-1148-1
Matley JK, Fisk AT, Dick TA (2013) The foraging ecology of Arctic cod (Boreogadus saida) during open water (July–August) in Allen Bay, Arctic Canada. Mar Biol 160:2993–3004. doi:10.1007/s00227-013-2289-2
Mills CE (2001) Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451:55–68
Möller H (1984) Reduction of a larval herring population by jellyfish predator. Science 224:621–622
Moulton LL, Tarbox KE (1987) Analysis of Arctic Cod Movements in the Beaufort Sea Nearshore Region, 1978-79. Arctic 40:43–49
Mutlu E (1996) Target strength of the common jellyfish (Aurelia aurita): a preliminary experimental study with a dual-beam acoustic system. ICES J Mar Sci 53:309–311
Purcell JE (2003) Predation on zooplankton by large jellyfish (Aurelia labiata, Cyanea capillata, Aequorea aequorea) in Prince William Sound, Alaska. Mar Ecol Prog Ser 246:137–152
Purcell JE (2005) Climate effects on formations of jellyfish and ctenophore blooms: a review. J Mar Biol Assoc UK 85:461–476
Purcell JE (2012) Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annu Rev Mar Sci 4:209–235
Purcell JE, Arai MN (2001) Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451:27–44
Purcell JE, Grover JJ (1990) Predation and food limitation as causes of mortality in larval herring at a spawning ground in British Columbia. Mar Ecol Prog Ser 59:55–67
Purcell JE, Siferd TD, Marliave JB (1987) Vulnerability of larval herring (Clupea harengus pallasi) to capture by the jellyfish Aequorea victoria. Mar Biol 94:157–162
Purcell JE, Uye S, Wen-Tseng L (2007) Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Mar Ecol Prog Ser 350:153–174. doi:10.3354/meps07093
Purcell JE, Hopcroft RR, Kosobokova KN, Whitledge TE (2010) Distribution, abundance, and predation effects of epipelagic ctenophores and jellyfish in the western Arctic Ocean. Deep Sea Res II 57:127–135
Samovol’kin VG (1974) Acoustic backscattering cross sections of small fish, crustaceans, and medusae as functions of their foreshortening. Oceanology 14:655–660
Schneider G, Behrends G (1998) Top–down control in a neritic plankton system by Aurelia aurita medusae—a summary. Ophelia 48:71–82
Siferd TD, Conover RJ (1992) Natural history of ctenophores in the Resolute Passage area of the Canadian High Arctic with special reference to Mertensia ovum. Mar Ecol Prog Ser 86:133–144
Welch HE, Crawford RE, Hop H (1993) Occurrence of Arctic cod (Boreogadus saida) schools and their vulnerability to predation in the Canadian High Arctic. Arctic 46:331–339
Acknowledgments
I acknowledge and thank all those scientists, technicians, students and residents of Resolute who assisted in the performance of this work. Over the years this project was supported by the Department of Fisheries and Oceans Canada, the Northern Oil and Gas Action Program, the Natural Sciences and Engineering Research Council of Canada, and Canada Foundation for Innovation (International Joint Ventures Fund) through the Ocean Tracking Network. The Polar Continental Shelf Program, Natural Resources Canada provided invaluable logistic support, including housing, transportation and operations with helicopters and Twin Otter aircraft. Comments by Jørgen Berge and three anonymous reviewers improved the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the special issue on the “Ecology of Arctic Gadids,” coordinated by Franz Mueter, Jasmine Nahrgang, John Nelson and Jørgen Berge.
Electronic supplementary material
Below is the link to the electronic supplementary material.
300_2015_1779_MOESM1_ESM.tif
Online Resource 1 General water temperature pattern in central Resolute Bay in summer 2013. Note the mixing of brackish meltwater into the upper 10 m when a breeze blew the ice cover out of the bay during 8/6–8/7. By 8/14/2013 subsequent insolation had warmed the upper 8 m above 0 °C and the preponderance of jellies had swarmed into this warmer layer of the water column. During a blizzard on 8/18/2013, upwelling mixed the water enough to lift colder water to the surface, resulting in a downward displacement of warm water into deeper reaches. Most gelatinous zooplankton were at 8–15 m (TIFF 2902 kb)
300_2015_1779_MOESM2_ESM.tif
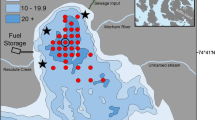
Online Resource 2 a. The day before a storm with ~60 km/h winds from the northeast, Arctic cod were numerous in a basin east of May Island in Allen Bay, Cornwallis Island. Red symbols indicate highest areal densities (301–2750 fish·m−2) determined by hydroacoustic echo integration along the cruise track (gaps in track = no data). b. The day after the storm, many Arctic cod were north of the basin in shallower water (same scale as above). Ice in the southern portion of the basin blocked a repeat survey there. It is postulated that upwelling during the storm, similar to that revealed in Online Resource 1, carried stinging jellyfish (C. capillata) into the basin and the fish moved into nearshore shallows to avoid nematocysts. Note the narrow channel (arrow) linking the basin to Allen Bay proper to the west. This is the same basin where Arctic cod shoaling was examined previously (Crawford and Jorgenson 1996) (TIFF 4649 kb)
Rights and permissions
About this article
Cite this article
Crawford, R.E. Occurrence of a gelatinous predator (Cyanea capillata) may affect the distribution of Boreogadus saida, a key Arctic prey fish species. Polar Biol 39, 1049–1055 (2016). https://doi.org/10.1007/s00300-015-1779-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1779-8