Abstract
Key message
A successful example of transposon deletion via CRISPR/Cas9-mediated genome editing suggests a novel alternative approach to plant breeding.
Abstract
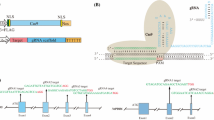
Transposition of transposable elements (TEs) can affect adjacent genes, leading to changes in genetic traits. Expression levels and patterns, splicing and epigenetic status, and function of genes located in, or near, the inserted/excised locus can be affected. Artificial modification of loci adjacent to TEs, or TEs themselves, by genome editing could mimic the translocation of TEs that occurs in nature, suggesting that it might be possible to produce novel plants by modification of TEs via genome editing. To our knowledge, there are no reports thus far of modification of TEs by genome editing in plants. In this study, we performed targeted deletion of the Tos17 retrotransposon, which is flanked at both ends by long terminal repeat (LTR) sequences, via genome editing in rice. We succeeded in targeted mutagenesis of the LTR, and targeted deletion between LTRs, in calli transformed with CRISPR/Cas9 vectors for the Tos17 LTR. Moreover, we also successfully obtained regenerated plants derived from transformed calli and plants homozygous for lacking Tos17 in the next generation. Taken together, our results demonstrate successful deletion of the Tos17 retrotransposon from the rice genome by targeted mutagenesis using CRISPR/Cas9. We believe that this strategy could be applied to other TEs in many plant species, providing a rapid breeding technology as an alternative means to re-activate expression of agronomically important genes that have been inactivated by TE insertion, especially in plants such as fruit trees, in which it is difficult to maintain parental agronomical traits by cross-breeding due to high heterozygosity.

Similar content being viewed by others
References
Cheng C, Daigen M, Hirochika H (2006) Epigenetic regulation of the rice retrotransposon Tos17. Mol Genet Genom 276:378–390
Chuong EB, Elde NC, Feschotte C (2017) Regulatory activities of transposable elements: from conflicts to benefits. Nat Rev Genet 18:71–86
Endo A, Masafumi M, Kaya H, Toki S (2016) Efficient targeted mutagenesis of rice and tobacco genomes using Cpf1 from Francisella novicida. Sci Rep 6:38169
Hirochika H, Sugimoto K, Otsuki Y, Tsugawa H, Kanda M (1996) Retrotransposons of rice involved in mutations induced by tissue culture. Proc Natl Acad Sci USA 93:7783–7788
Kwon YI, Abe K, Osakabe K, Endo M, Nishizawa-Yokoi A, Saika H, Shimada H, Toki S (2012) Overexpression of OsRecQl4 and/or OsExo1 enhances DSB-induced homologous recombination in rice. Plant Cell Physiol 53:2142–2152
Lisch D (2013) How important are transposons for plant evolution? Nat Rev Genet 14:49–61
Mikami M, Toki S, Endo M (2016) Precision targeted mutagenesis via Cas9 paired nickases in rice. Plant Cell Physiol 57:1058–1068
Nishizawa-Yokoi A, Cermak T, Hoshino T, Sugimoto K, Saika H, Mori A, Osakabe K, Hamada M, Katayose Y, Starker C, Voytas DF, Toki S (2016) A defect in DNA ligase4 enhances the frequency of TALEN-mediated targeted mutagenesis in rice. Plant Physiol 170:653–666
Siebert R, Puchta H (2002) Efficient repair of genomic double-strand breaks by homologous recombination between directly repeated sequences in the plant genome. Plant Cell 14:1121–1131
Zhou H, Liu B, Weeks DP, Spalding MH, Yang B (2014) Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res 42:10903–10914
Acknowledgements
We thank Mr. Masafumi Mikami and Dr. Ayako Nishizawa-Yokoi for critical discussion about targeted mutagenesis in rice; Drs. Tomoki Hoshino and Kazuhiko Sugimoto for providing CelI enzyme and Dr. Helen Rothnie for English editing. We also thank Kiyoko Amagai, Rieko Aoto, Akemi Nagashii and Fukuko Suzuki for technical assistance. This work was supported by the Ministry of Agriculture, Forestry and Fisheries of Japan, the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry to H.S., and the Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP), “Technologies for creating next-generation agriculture, forestry and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO) to S.T.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Neal Stewart.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saika, H., Mori, A., Endo, M. et al. Targeted deletion of rice retrotransposon Tos17 via CRISPR/Cas9. Plant Cell Rep 38, 455–458 (2019). https://doi.org/10.1007/s00299-018-2357-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-018-2357-7