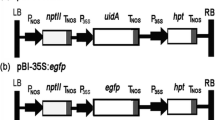
Abstract
An efficient genetic transformation procedure using a recombinant green fluorescent protein (GFP) has been developed in Hevea brasiliensis clone PB260. Transformation experiments have been performed using an Agrobacterium tumefaciens binary vector harbouring both uidA and S65T-GFP reporter genes in order to compare selection methods using glucuronidase assay (GUS activity) and paromomycin resistance, GFP activity and paromomycin resistance, or GFP activity only. At transient level, the number of spots showing GUS or GFP activities was similar for 4 and 5 days after coculture. After selection, stable transformation events were observed and led to the establishment of transgenic callus lines. A higher number of lines were generated with GFP selection compared to the GUS one. GFP selection is less time-consuming in terms of callus subculturing, and offers the possibility of producing antibiotic resistance marker-free transgenic plants.


Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- BAP:
-
Benzylaminopurine
- MM:
-
Maintenance medium
- PM:
-
Pre-culture medium
- DM:
-
Decontamination medium
- 3,4-D:
-
3,4-Diclorophenoxyacetic acid
- GUS:
-
β-Glucuronidase
- GFP:
-
Green fluorescent protein
References
Ahlandsberg S, Sathish P, Sun C, Jansson C (1999) Green fluorescent protein as a reporter system in the transformation of barley cultivars. Physiol Plant 107:194–200
Arokiaraj P, Jones H, Jaafar H, Coomber S, Charlwood B (1996) Agrobacterium mediated transformation of Hevea anther calli and their regeneration into plantlets. J Nat Rubber Res 11:77
Blanc G, Baptiste C, Oliver G, Martin F, Montoro P (2006) Efficient Agrobacterium tumefaciens-mediated transformation of embryogenic calli and regeneration of Hevea brasiliensis Müll Arg. plants. Plant Cell Rep 24:724–733
Carron M-P, Etienne H, Lardet L, Campagna S, Perrin Y, Leconte A, Chaine C (1995) Somatic embryogenesis in rubber (Hevea brasiliensis Müll. Arg.). In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants. Kluwer, Dordrecht, pp 117–136
Cervera M, Navarro A, Navarro L, Pena L (2008) Production of transgenic adult plants from clementine mandarin by enhancing cell competence for transformation and regeneration. Tree Physiol 28:55–66
Cho M-J, Ha CD, Lemaux PG (2000) Production of transgenic tall fescue and red fescue plants by particle bombardment of mature seed-derived highly regenerative tissues plant Cell Rep 19:1084–1089
Elliott A, Campbell J, Dugdale B, Brettell R, Grof C (1999) Green-fluorescent protein facilitates rapid in vivo detection of genetically transformed plant cells. Plant Cell Rep 18:707–714
Engelmann F, Lartaud M, Chabrillange N, Carron M, Etienne H (1997) Cryopreservation of embryogenic calli of two commercial clones of Hevea brasiliensis. Cryo Lett 18:107–116
Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25:989–994
Haseloff J, Amos B (1995) GFP in plants. Trends Genet 11:328–329
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Heim R, Tsien RY (1996) Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol 6:178–182
Jayashree R, Rekha K, Venkatachalam P, Uratsu SL, Dandekar AM, Kumari Jayasree P, Kala RG, Priya P, Sushma Kumari S, Sobha S, Ashokan MP, Sethuraj MR, Thulaseedharan A (2003) Genetic transformation and regeneration of rubber tree (Hevea brasiliensis Müll. Arg) transgenic plants with a constitutive version of an anti-oxidative stress superoxide dismutase gene. Plant Cell Rep 22:201–209
Kim HJ, Kato N, Kim S, Triplett B (2008) Cu/Zn superoxide dismutases in developing cotton fibers: evidence for an extracellular form. Planta 228:281–292
Lardet L, Piombo G, Oriol M, Dechamp E, Carron MP (1999) Relation between biochemical characteristics and conversion ability in Hevea brasiliensis zygotic and somatic embryos. Can J Bot 77:1168–1177
Lardet L, Martin F, Dessailly F, Carron MP, Montoro P (2007) Effect of exogenous calcium on post-thaw growth recovery and subsequent plant regeneration of cryopreserved embryogenic calli of Hevea brasiliensis (Müll. Arg.). Plant Cell Rep 26:559–569
Lardet L, Desailly F, Carron MP, Rio MA, Ferrière N, Montoro P (2009) Secondary somatic embryogenesis in Hevea brasiliensis (Müll. Arg.): an alternative process for long-term somatic embryogenesis. J Rubber Res 12(4):215–228
Leclercq J, Ranty B, Sanchez-Ballesta MT, Li Z, Jones B, Jauneau A, Pech JC, Latche A, Ranjeva R, Bouzayen M (2005) Molecular and biochemical characterization of LeCRK1, a ripening-associated tomato CDPK-related kinase. J Exp Bot 56:25–35
Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ (1999) Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun 260:712–717
Maximova S, Miller C, Antunez de Mayolo G, Pishak S, Young A, Guiltinan MJ (2003) Stable transformation of Theobroma cacao L. and influence of matrix attachment regions on GFP expression. Plant Cell Rep 21:872–883
Montoro P, Teinseree N, Rattana W, Kongsawadworakul P, Michaux-Ferrière N (2000) Effect on exogenous calcium on Agrobacterium tumefaciens-mediated gene transfer in Hevea brasiliensis (rubber tree) friable call. Plant Cell Rep 19:851–855
Montoro P, Rattana W, Pujade-Renaud V, Michaux-Ferriere N, Monkolsook Y, Kanthapura R, Adunsadthapong S (2003) Production of Hevea brasiliensis transgenic embryogenic callus lines by Agrobacterium tumefaciens: roles of calcium. Plant Cell Rep 21:1095–1102
Montoro P, Lagier S, Baptiste C, Marteaux B, Pujade-Renaud V, Leclercq J, Alemanno L (2008) Expression of the HEV2.1 gene promoter in transgenic Hevea brasiliensis. Plant Cell Tissue Organ Cult 94:55–63
Pang SZ, DeBoe DL, Wan Y, Ye G, Layton JG, Neher MK, Armstrong CL, Fry JE, Hinchee MA, Fromm ME (1996) An improved green fluorescent protein gene as a vital marker in plants. Plant Physiol 112:893–900
Petri C, Wang H, Alburquerque N, Faize M, Burgos L (2008) Agrobacterium-mediated transformation of apricot (Prunus armeniaca L.) leaf explants. Plant Cell Rep 27:1317–1324
Rattana W, Teinseree N, Tadakittisarn S, Pujade-Renaud V, Monkolsook Y, Montoro P (2001) Characterization of factors involved in tissue growth recovery and stability of GUS activity in rubber tree (Hevea brasiliensis) friable calli transformed by Agrobacterium tumefaciens. Thai J Agric Sci 34:195–204
Risterucci AM, Grivet L, N’Goran JKA, Pieretti I, Flament MH, Lanaud C (2000) A high-density linkage map of Theobroma cacao L. Theor Appl Genet 101:948–955
Saika H, Toki S (2009) Visual selection allows immediate identification of transgenic rice calli efficiently accumulating transgene products. Plant Cell Rep 28:619–626
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. In: Harbor CS (ed) A laboratory manual. Cold Spring Harbor Laboratory Press, New York
Santos-Rosa M, Poutaraud A, Merdinoglu D, Mestre P (2008) Development of a transient expression system in grapevine via agro-infiltration. Plant Cell Rep 27:1053–1063
Shimomura O, Johnson FH, Saiga Y (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol 59:223–239
Stewart CN Jr (2001) The utility of green fluorescent protein in transgenic plants. Plant Cell Rep 20:376–382
Tian L, Levee V, Mentag R, Charest PJ, Seguin A (1999) Green fluorescent protein as a tool for monitoring transgene expression in forest tree species. Tree Physiol 19:541–546
Urbanus SL, de Folter S, Shchennikova AV, Kaufmann K, Immink RG, Angenent GC (2009) In planta localisation patterns of MADS domain proteins during floral development in Arabidopsis thaliana. BMC Plant Biol 9:5
Vain P, Worland B, Kohli A, Snape J, Christou P (1998) The green fluorescent protein (GFP) as a vital screenable marker in rice transformation. Theor Appl Genet 96:164–169
Vancanneyt G, Schmidt R, O’Connor-Sanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220:245–250
Watanabe K, Yamano Y, Murata K, Kimura A (1986) The nucleotide sequence of the gene for gamma-glutamylcysteine synthetase of Escherichia coli. Nucleic Acids Res 14:4393–4400
Yancheva SD, Shlizerman LA, Golubowicz S, Yabloviz Z, Perl A, Hanania U, Flaishman MA (2006) The use of green fluorescent protein (GFP) improves Agrobacterium-mediated transformation of ‘Spadona’ pear (Pyrus communis L.). Plant Cell Rep 25:183–189
Acknowledgments
This work was supported by the Institut Français pour le Caoutchouc. The authors thank Dr. J.-C. Breitler for providing the GFP-intron cassette and Dr. R. Jefferson (CAMBIA, Australia) for providing the pCAMBIA vectors and the EHA105 A. tumefaciens strain. The authors would also like to thank André Clément-Demange for the statistical analyses; and F.-C. Baurens, P. Biggins and T. Tranberger for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Jouanin.
Rights and permissions
About this article
Cite this article
Leclercq, J., Lardet, L., Martin, F. et al. The green fluorescent protein as an efficient selection marker for Agrobacterium tumefaciens-mediated transformation in Hevea brasiliensis (Müll. Arg). Plant Cell Rep 29, 513–522 (2010). https://doi.org/10.1007/s00299-010-0840-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0840-x