Abstract
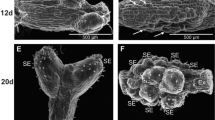
Relationships between mineral uptake and tobacco shoot organogenesis were investigated during three morphogenic phases: phase 1, days 0–10, pre-meristem formation; phase 2, days 10–20, meristem initiation and formation; and phase 3, days 20–35, growth and differentiation of induced meristems into leafy shoots. The mineral content of both shoot-forming (SF) and non-shoot-forming (NSF) media was examined over the 35-day culture period. Both SF and NSF explants rapidly consumed iron during phase 1. Nitrate uptake in SF explants was high and independent of explant growth during phases 1 and 2, but greatest and strongly correlated with growth during phase 3. Phosphorus uptake was highest in SF explants during phases 2 and 3, and correlated with explant growth. Uptake of potassium, calcium and sulphur was strongly associated with explant growth during phase 3 whereas magnesium uptake was only poorly correlated with growth. Results from this study indicate that particular minerals may have an important role in regulating development as well as generally supporting growth.



Similar content being viewed by others
Abbreviations
- BA :
-
N 6-Benzyladenine
- NSF :
-
Non-shoot forming
- RGR :
-
Relative growth rate
- SF :
-
Shoot forming
References
Bellamy AR, Bieleski RL (1966) Some salt-uptake and tissue aging phenomena studied with cultured tobacco cells. Aust J Biol Sci 19:23–36
Bellini C, Chupeau M, Gervais M, Vastra G, Chupeau Y (1990) Importance of myo-inositol, calcium, and ammonium for the viability and division of tomato (Lycopersicon esculentum) protoplasts. Plant Cell Tissue Org Cult 18:221–226
Brown DCW, Thorpe TA (1986) Plant regeneration by organogenesis. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol 3. Academic, New York, pp 49–65
Cousson A, Tran Thanh Van K (1993) Influence of ionic composition of the culture medium on de novo flower formation in tobacco thin cell layers. Can J Bot 71:506–511
Curtis WR, Hasegawa M, Emery AH (1991) Modelling linear and variable growth in phosphate limited suspension cultures of opium poppy. Biotechnol Bioeng 38:371–379
Debergh PC, De Riek J, Matthys D (1994) Nutrient supply and growth of plants in culture. In: Lumsden PJ, Nicholas JR, Davies WJ (eds) Physiology, growth and development of plants in culture. Kluwer, Dordrecht
Eklund L, Eliasson L (1990) Effects of calcium concentration on cell wall synthesis. J Exp Bot 41:863
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
George EF, Puttock DJM, George HJ (1987) Plant culture media, vol 1. Formulations and uses. Exegetics, Edington, UK
George EF, Puttock DJM, George HJ (1988) Plant culture media, vol 2. Commentary and analysis. Exegetics, Edington, UK
Hardy EL, Thorpe TA (1990) Nitrate assimilation in shoot-forming tobacco callus cultures. In Vitro Cell Dev Biol 26:525–530
Hunt R (1982) The Plant growth curves, the functional approach to plant growth analysis. Arnold, London
Hush JM, Overall RL, Newman IA (1991) A calcium influx preceded organogenesis in Graptopetalum. Plant Cell Environ 14:657–665
Joy RW, Bender L, Thorpe TA (1994) Nitrogen metabolism in cultured cotyledons of Pinus radiata during de novo organogenesis. Physiol Plant 92:681–688
Kato A, Fukasawa A, Shimizu Y, Soh Y, Nagai S (1977) Requirements of PO4 3-, NO3 -, SO4 2-, K+, and Ca2+ for the growth of tobacco cells in suspension culture. J Ferment Technol 55:207
Leifert C, Murphy KP, Lumsden PJ (1995) Mineral and carbohydrate nutrition of plant cell and tissue cultures. CRC Crit Rev Plant Sci 14:83–109
Lumsden PJ, Pryce S, Leifert C (1990) Effect of mineral nutrition on the growth and multiplication of in vitro cultured plants. In: Nijkamp HJJ, Van Der Plas LHW, Van Aartijk J (eds) Progress in plant cellular and molecular biology. Kluwer, Dordrecht, pp. 108–113
Marschner M (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Marshall B, Porter JR (1991) Concepts of nutritional and environmental interactions determining plant productivity. In: Porter JR, Lawlor DW (eds) Plant growth: interactions with nutrients and environments. Cambridge University Press, Cambridge, pp 99–124
Mengel K, Kirkby EA (1987) Principles of plant nutrition. International Potash Institute, Switzerland
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ramage CM, Williams RR (2002a) Mineral nutrition and plant morphogenesis. In Vitro Cell Dev Biol Plant 38:116–124. DOI 10.1079/IVP2002269
Ramage CM, Williams RR (2002b) Inorganic nitrogen requirements during shoot organogenesis in tobacco leaf discs. J Exp Bot 53:1437–1443
Saunders MJ (1992) Cytokinin signal transduction through Ca2+ in mosses. In: Karssen CM, Loon van LC, Vreugdenhil D (eds) Progress in plant growth regulation. Proceedings of the 14th International Conference on Plant Growth Substances, Amsterdam 21–26 July 1991. Kluwer, Dordrecht, pp 65–72
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204
Schenk N, Hsiao KC, Bornman CH (1991) Avoidance of precipitation and carbohydrate breakdown in autoclaved plant tissue culture media. Plant Cell Rep 10:115–119
Schmitz U, Lörz H (1990a) Nutrient uptake in suspension cultures of Graminae. I. Development of an assay system. Plant Sci 66:87–94
Schmitz U, Lörz H (1990b) Nutrient uptake in suspension cultures of Graminae. II. Suspension cultures of rice. Plant Sci 66:87–94
Skoog F (1944) Growth and organ formation in tobacco tissue cultures. Am J Bot 31:19–24
Sokal RR, Rohlf FJ (1981) Biometry, the principles and practice of statistics in biological research, 2nd edn. Freeman
Tanimoto S, Harada H (1986) Involvement of calcium in adventitious bud initiation in Torenia stem segments. Plant Cell Physiol 27:1–10
Tran Thanh Van M (1973) Direct flower neo-formation from superficial tissue of small explants of Nicotiana tabacum L. Planta 115:87–92
Ueki K, Sato S (1971) Effect of inorganic phosphate on the extracellular acid phosphatase activity of tobacco cells cultured in vitro. Plant Physiol 24:506
Veliky IA, Rose D, Zink MW (1976) Uptake of magnesium by suspension cultures of plant cells (Ipomoea sp.). Can J Bot 55:1143–1147
Williams RR (1993) Mineral nutrition in vitro—A mechanistic approach. Aust J Bot 41:237–251
Williams RR (1995) The chemical micro-environment. In: Aitken-Christie J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Kluwer, Dordrecht, pp 405–439
Acknowledgements
We would like to thank the technical staff of the School of Agronomy and Horticulture. This research was supported by a University of Queensland Collaborative Research Grant number 97/QUALR3018G.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.F. Rose
Rights and permissions
About this article
Cite this article
Ramage, C.M., Williams, R.R. Mineral uptake in tobacco leaf discs during different developmental stages of shoot organogenesis. Plant Cell Rep 21, 1047–1053 (2003). https://doi.org/10.1007/s00299-003-0628-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0628-3