Abstract
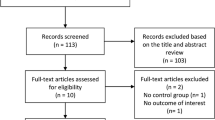
We performed a systematic review and meta-analysis of studies evaluating vascular function in patients with JIA. Relevant literature published from 1st January 1965 to 1st March 2022 was searched systematically utilizing PubMed, Web of Science, and Embase databases. Observational studies were included—patients with JIA (classified according to the International League of Associations for Rheumatology criteria) were included as cases (study population) and age/sex-matched healthy participants as controls (comparator group). Outcome measures were differences in non-invasive parameters of vascular function. Online Population, Intervention, Comparison, Outcomes Portal was used for deduplication of studies and data extraction. Review Manager, Comprehensive Meta-analysis, and Meta-Essential softwares were used for data synthesis/analysis (encompassing data pooling and evaluation of heterogeneity and publication bias). Newcastle–Ottawa Scale and GRADEpro GDT software were utilized to assess study quality and certainty of evidence, respectively. Of 338 citations, 17 observational studies with 1423 participants (cases = 757, controls = 666) were included. Carotid intima-media thickness (CIMT) was higher [mean difference (MD) 0.02 mm {95% confidence interval (CI) 0.01–0.04}, p = 0.0006, I2 = 69%] in patients with JIA. Besides, decreased flow-mediated dilatation (FMD) [MD − 2.18% {95%CI − 3.69– − 0.68}, p = 0.004, I2 = 73%] was also observed. Results of studies assessing pulse wave velocity or arterial stiffness could not be pooled due to significant methodological variations. A ‘very low’ certainty of evidence suggests the presence of vascular dysfunction in JIA. Future longitudinal studies are required to determine whether altered CIMT and FMD in patients with JIA translate to an enhanced risk of (adverse) clinical cardiovascular events. PROSPERO (CRD42022323752).




Similar content being viewed by others
Data availability
Relevant data utilized in the preparation of this manuscript are provided in the main manuscript and supplementary tables/figures. Additional data underlying this article will be shared on reasonable request to the corresponding author.
References
Nowbar AN, Gitto M, Howard JP, Francis DP, Al-Lamee R (2019) Mortality from ischemic heart disease: analysis of data from the World Health Organization and coronary artery disease risk factors from NCD Risk Factor Collaboration. Circ Cardiovasc Qual Outcomes. https://doi.org/10.1161/CIRCOUTCOMES.118.005375
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F et al (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364:937–952. https://doi.org/10.1016/S0140-6736(04)17018-9
Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J (2011) Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol 27:174–182. https://doi.org/10.1016/j.cjca.2010.12.040
Liao KP (2017) Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc 27:136–140. https://doi.org/10.1016/j.tcm.2016.07.006
Kostopoulou M, Nikolopoulos D, Parodis I, Bertsias G (2020) Cardiovascular disease in systemic lupus erythematosus: Recent data on epidemiology, risk factors and prevention. Curr Vasc Pharmacol 18:549–565. https://doi.org/10.2174/1570161118666191227101636
Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 59:1690–1697. https://doi.org/10.1002/art.24092
England BR, Thiele GM, Anderson DR, Mikuls TR (2018) Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. https://doi.org/10.1136/bmj.k1036
Zaripova LN, Midgley A, Christmas SE, Beresford MW, Baildam EM, Oldershaw RA (2021) Juvenile idiopathic arthritis: from aetiopathogenesis to therapeutic approaches. Pediatr Rheumatol Online J 19:135. https://doi.org/10.1186/s12969-021-00629-8
Dave M, Rankin J, Pearce M, Foster HE (2020) Global prevalence estimates of three chronic musculoskeletal conditions: club foot, juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. Pediatr Rheumatol Online J 18:49. https://doi.org/10.1186/s12969-020-00443-8
Prakken B, Albani S, Martini A (2011) Juvenile idiopathic arthritis. Lancet 377:2138–2149. https://doi.org/10.1016/s0140-6736(11)60244-4
Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R et al (2019) Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol 46:190–197. https://doi.org/10.3899/jrheum.180168
Jednacz E, Rutkowska-Sak L (2012) Atherosclerosis in juvenile idiopathic arthritis. Mediators Inflamm 2012:1–5. https://doi.org/10.1155/2012/714732
Bohr A-H, Fuhlbrigge RC, Pedersen FK, de Ferranti SD, Müller K (2016) Premature subclinical atherosclerosis in children and young adults with juvenile idiopathic arthritis. A review considering preventive measures. Pediatr Rheumatol Online J 14:3. https://doi.org/10.1186/s12969-015-0061-5
Anderson JH, Anderson KR, Aulie HA, Crowson CS, Mason TG, Ardoin SP et al (2016) Juvenile idiopathic arthritis and future risk for cardiovascular disease: a multicenter study. Scand J Rheumatol 45:299–303. https://doi.org/10.3109/03009742.2015.1126345
Coulson EJ, Ng W-F, Goff I, Foster HE (2013) Cardiovascular risk in juvenile idiopathic arthritis. Rheumatology (Oxford) 52:1163–1171. https://doi.org/10.1093/rheumatology/ket106
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. https://doi.org/10.1136/bmj.n71
PICO Portal (2022) Identifying duplicates with PICO Portal. Available from: https://picoportal.org/2022/02/01/identifying-duplicates-with-pico-portal/
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Review Manager (RevMan), version 5.3, Copenhagen (2014) The Nordic 18 Cochrane Centre. The Cochrane Collaboration. Available from: https://training.cochrane.org/online-learning/core-software/revman
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors (2022) Cochrane handbook for systematic reviews of interventions version 6.3. Available from: https://training.cochrane.org/handbook
Suurmond R, van Rhee H, Hak T (2017) Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods 8:537–553. https://doi.org/10.1002/jrsm.1260
Schünemann H, Brożek J, Guyatt G, Oxman A, editors (2013) GRADE Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group. Available from: https://gdt.gradepro.org/app/handbook/handbook.html
Breda L, Di Marzio D, Giannini C, Gaspari S, Nozzi M, Scarinci A et al (2013) Relationship between inflammatory markers, oxidant-antioxidant status and intima-media thickness in prepubertal children with juvenile idiopathic arthritis. Clin Res Cardiol 102:63–71. https://doi.org/10.1007/s00392-012-0496-3
Ebraheem MF, Sallam RA-ER, Mohsen MA, El-Kady BA, El-Hawary GE, Baiomy AA (2019) Vascular cell adhesion molecule-1 (VCAM-1), flow mediated dilatation (FMD) and carotid intima media thickness (IMT) in children with juvenile idiopathic arthritis: relation to disease activity, functional status and fatigue. Egypt Rheumatol 41:145–150. https://doi.org/10.1016/j.ejr.2018.05.007
Evensen K, Aulie HA, Rønning OM, Flatø B, Russell D (2016) Carotid atherosclerosis in adult patients with persistently active juvenile idiopathic arthritis compared with healthy controls. J Rheumatol 43:810–815. https://doi.org/10.3899/jrheum.150499
Hussain KS, Gulati R, Satheesh S, Negi VS (2021) Early-onset subclinical cardiovascular damage assessed by non-invasive methods in children with juvenile idiopathic arthritis: analytical cross-sectional study. Rheumatol Int 41:423–429. https://doi.org/10.1007/s00296-020-04689-z
Ilisson J, Zagura M, Zilmer K, Salum E, Heilman K, Piir A et al (2015) Increased carotid artery intima-media thickness and myeloperoxidase level in children with newly diagnosed juvenile idiopathic arthritis. Arthritis Res Ther 17:180. https://doi.org/10.1186/s13075-015-0699-x
Jednacz E, Rutkowska-Sak L (2015) Assessment of the body composition and parameters of the cardiovascular risk in juvenile idiopathic arthritis. BioMed Res Int. https://doi.org/10.1155/2015/619023
Mani P, Uno K, Duong M, Wolski K, Spalding S, Husni ME et al (2016) HDL function and subclinical atherosclerosis in juvenile idiopathic arthritis. Cardiovasc Diagn Ther 6:34–43. https://doi.org/10.3978/j.issn.2223-3652.2015.12.14
Aranda-Valera IC, Arias de la Rosa I, Roldán-Molina R, Ábalos-Aguilera MDC, Torres-Granados C, Patiño-Trives A et al (2020) Subclinical cardiovascular risk signs in adults with juvenile idiopathic arthritis in sustained remission. Pediatr Rheumatol Online J 18:59. https://doi.org/10.1186/s12969-020-00448-3
Vlahos AP, Theocharis P, Bechlioulis A, Naka KK, Vaka lis K, Papamichael ND et al (2011) Changes in vascular function and structure in juvenile idiopathic arthritis. Arthritis Care Res 63:1736–1744. https://doi.org/10.1002/acr.20613
Satija M, Yadav TP, Sachdev N, Chhabra A, Jahan A, Dewan V (2014) Endothelial function, arterial wall mechanics and intima media thickness in juvenile idiopathic arthritis. Clin Exp Rheumatol 32:432–439
Turoňová L, Kubejová K, Vorčáková K, Ďurdík P, Péčová T, Martinásková K (2018) Endothelial dysfunction in children with juvenile psoriatic arthritis. Acta Med (Hradec Kralove) 61:79–85
Argyropoulou MI, Kiortsis DN, Daskas N, Xydis V, Mavridis A, Efremidis SC et al (2003) Distensibility and pulse wave velocity of the thoracic aorta in patients with juvenile idiopathic arthritis: an MRI study. Clin Exp Rheumatol 21:794–797
Aulie HA, Selvaag AM, Günther A, Lilleby V, Molberg Ø, Hartmann A et al (2015) Arterial haemodynamics and coronary artery calcification in adult patients with juvenile idiopathic arthritis. Ann Rheum Dis 74:1515–1521. https://doi.org/10.1136/annrheumdis-2013-204804
Picarelli MM, Danzmann LC, Grun LK, Júnior NTR, Lavandovsky P, Guma FTCR et al (2017) Arterial stiffness by oscillometric device and telomere lenght in juvenile idiopathic arthritis with no cardiovascular risk factors: a cross-sectional study. Pediatr Rheumatol Online J 15:34. https://doi.org/10.1186/s12969-017-0165-1
Sozeri B, Atikan BY, Ozdemir K, Mir S (2016) Assessment of vascular function in systemic onset juvenile idiopathic arthritis. Clin Rheumatol 35:1699–1703. https://doi.org/10.1007/s10067-016-3254-5
Dedeoglu R, Yildiz M, Karagozlu F, Oztunc F, Ulug N, Akdeniz B et al (2020) Unexpected increase of aortic stiffness in juvenile spondyloarthropathies. Cardiol Young 30:1806–1814. https://doi.org/10.1017/s1047951120002796
Aulie HA, Estensen M-E, Selvaag AM, Lilleby V, Flatø B, Aakhus S (2018) Arterial properties in adults with long-lasting active juvenile idiopathic arthritis compared to healthy controls. Pediatr Rheumatol Online J 16:85. https://doi.org/10.1186/s12969-018-0302-5
Salonen JT, Salonen R (1991) Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb 11:1245–1249. https://doi.org/10.1161/01.atv.11.5.1245
Øygarden H (2017) Carotid intima-media thickness and prediction of cardiovascular disease. J Am Heart Assoc. https://doi.org/10.1161/jaha.116.005313
Wang P, Guan S-Y, Xu S-Z, Li H-M, Leng R-X, Li X-P et al (2016) Increased carotid intima-media thickness in rheumatoid arthritis: an update meta-analysis. Clin Rheumatol 35:315–323
van Sijl AM, Peters MJ, Knol DK, de Vet HC, Gonzalez-Gay MA, Smulders YM et al (2011) Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 40:389–397. https://doi.org/10.1016/j.semarthrit.2010.06.006
Tyrrell PN, Beyene J, Feldman BM, McCrindle BW, Silverman ED, Bradley TJ (2010) Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 30:1014–1026. https://doi.org/10.1161/atvbaha.109.198424
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA et al (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. J Am Coll Cardiol 39:257–265. https://doi.org/10.1016/s0735-1097(01)01746-6
Di Minno MND, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R et al (2015) Clinical assessment of endothelial function in patients with rheumatoid arthritis: a meta-analysis of literature studies. Eur J Intern Med 26:835–842. https://doi.org/10.1016/j.ejim.2015.10.016
Kaplan MJ (2010) Cardiovascular complications of rheumatoid arthritis: assessment, prevention, and treatment. Rheum Dis Clin North Am 36:405–426. https://doi.org/10.1016/j.rdc.2010.02.002
Chan SY, Mancini GBJ, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A (2003) The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol 42:1037–1043. https://doi.org/10.1016/s0735-1097(03)00927-6
Kim H-L, Kim S-H (2019) Pulse wave velocity in atherosclerosis. Front Cardiovasc Med 6:41. https://doi.org/10.3389/fcvm.2019.00041
Funding
None.
Author information
Authors and Affiliations
Contributions
PKP: conception of idea and design of the study; acquisition, analysis, and interpretation of data; drafted, edited, and critically revised the manuscript. AZB, RN: acquisition, analysis, and interpretation of data; drafted, edited, and critically revised the manuscript. AA, RRD: designed the study; acquisition, analysis, and interpretation of data; edited and critically revised the manuscript. PR, DB: acquisition, analysis, and interpretation of data; edited and critically revised the manuscript. All co-authors approve the final version of the manuscript and take full responsibility for the integrity and accuracy of all aspects of the study.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval and informed consent
As this study pertains to literature review and analysis of previously published data, specific ethics approval is not mandated.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
296_2022_5255_MOESM1_ESM.tif
Supplementary file1 (TIF 199 KB) Supplementary Fig. S1: Funnel plots assessing the publication bias for various outcome measures of vascular function. A: No publication bias was observed for studies assessing CIMT in patients with JIA as compared to controls. The corresponding p-values of Egger regression and Begg & Mazumdar tests were 0.2 and 0.6, respectively; B: lack of publication bias was also observed for studies evaluating FMD in patients with JIA as compared to controls. The corresponding p-values of Egger regression and Begg & Mazumdar tests were 0.6 and 0.5, respectively.
296_2022_5255_MOESM2_ESM.tif
Supplementary file2 (TIF 134 KB) Supplementary Fig. S2: Regression plots assessing the correlation between heterogeneity and individual covariates. A: In studies comparing CIMT of patients with JIA to controls, age at enrolment significantly correlated (p=0.004) with the underlying heterogeneity; B: although none of the covariates included associated significantly with heterogeneity in studies evaluating FMD, levels of serum triglycerides showed a trend towards statistical significance (p=0.08).
296_2022_5255_MOESM3_ESM.tif
Supplementary file3 (TIF 2181 KB) Supplementary Fig. S3: Forest plots comparing CIMT between various subgroups of patients with JIA. A: Patients with sJIA and non-systemic JIA (including oligoarthritis and polyarthritis) had comparable CIMT; B: similar CIMT was observed in patients with oligoarthritis and polyarthritis; C: no significant difference in CIMT was noted when patients with active disease were compared to patients with inactive disease.
296_2022_5255_MOESM4_ESM.tif
Supplementary file4 (TIF 1496 KB) Supplementary Fig. S4: Forest plots comparing FMD between various subgroups of patients with JIA. A: Patients with sJIA and non-systemic JIA (including oligoarthritis and polyarthritis) had comparable FMD; B: similar FMD was observed in patients with oligoarthritis and polyarthritis.
296_2022_5255_MOESM5_ESM.tif
Supplementary file5 (TIF 782 KB) Supplementary Fig. S5: Similar CIMT was noted in patients with JIA and controls in the studies wherein the final measure of intima-media thickness also included bilateral carotid bulbs plus/minus bilateral internal carotid arteries (in addition to common carotid arteries).
296_2022_5255_MOESM6_ESM.tif
Supplementary file6 (TIF 764 KB) Supplementary Fig. S6: Decreased FMD is noted in patients with JIA when results of studies with similar levels of traditional cardiovascular risk factors in the 2 groups (patients and controls) are pooled.
Supplementary file8 (DOCX 40 KB) Supplementary Table S2: PRISMA 2020 for main manuscripts checklist.
296_2022_5255_MOESM9_ESM.docx
Supplementary file9 (DOCX 30 KB) Supplementary Table S3: Protocol(s) used in various studies to evaluate carotid intima-media thickness in patients with JIA.
296_2022_5255_MOESM10_ESM.docx
Supplementary file10 (DOCX 36 KB) Supplementary Table S4: Quality of the studies included according to the Modified Newcastle-Ottawa Scale.
296_2022_5255_MOESM11_ESM.docx
Supplementary file11 (DOCX 17 KB) Supplementary Table S5: Grade of evidence of altered CIMT and FMD in patients with JIA as compared to controls.
296_2022_5255_MOESM12_ESM.docx
Supplementary file12 (DOCX 35 KB) Supplementary Table S6: Disease and treatment characteristics of studies evaluating vascular function in patients with juvenile idiopathic arthritis in comparison to controls. Traditional cardiovascular risk factors (and related parameters) that were significantly different between cases and controls have also been detailed briefly.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patra, P.K., Banday, A.Z., Asghar, A. et al. Vascular dysfunction in juvenile idiopathic arthritis: a systematic review and meta-analysis. Rheumatol Int 43, 33–45 (2023). https://doi.org/10.1007/s00296-022-05255-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05255-5