Abstract
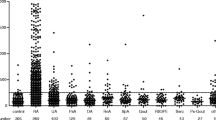
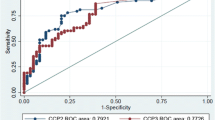
To evaluate whether C-reactive protein (CRP), including variation within the normal range, is predictive of long-term disease outcome in Juvenile Idiopathic Arthritis (JIA). Consecutive patients with newly diagnosed JIA were included prospectively from defined geographic areas of the Nordic countries from 1997 to 2000. Inclusion criteria were availability of a baseline serum sample within 12 months after disease onset and 8-year clinical assessment data. Systemic onset JIA was not included. CRP was measured by high-sensitive ELISA (detection limit of 0.2 mg/l). One hundred and thirty participants with a median follow-up time of 97 months (range 95–100) were included. At follow-up, 38% of the patients were in remission off medication. Absence of remission was associated with elevated level of CRP at baseline (odds ratio (OR) 1.33, confidence interval (CI) 1.08–1.63, p = 0.007). By applying a cutoff at the normal upper limit (>10 mg/l), the risk of not achieving remission was increased to an OR of 8.60 (CI 2.98–24.81, p < 0.001). Variations of CRP within the normal range had no predictive impact on disease activity at follow-up. Baseline levels of ESR were available in 80 patients (61%) and elevated ESR was associated with absence of remission in a multivariable logistic regression analysis (OR 2.32, CI 1.35-4.00, p = 0.002). This results of this study indicate that baseline CRP concentrations above 10 mg/l are predictive of a poor outcome at 8-year follow-up. We could not demonstrate any predictive value of CRP variations within the normal range.


Similar content being viewed by others
References
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J et al (2004) International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31(2):390–392
Berntson L, Andersson GB, Fasth A, Herlin T, Kristinsson J, Lahdenne P et al (2003) Incidence of juvenile idiopathic arthritis in the Nordic countries. A population based study with special reference to the validity of the ILAR and EULAR criteria. J Rheumatol 30(10):2275–2282
Nordal E, Zak M, Aalto K, Berntson L, Fasth A, Herlin T et al (2011) Ongoing disease activity and changing categories in a long-term nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum 63(9):2809–2818
Zak M, Pedersen FK (2000) Juvenile chronic arthritis into adulthood: a long-term follow-up study. Rheumatology (Oxf) 39(2):198–204
van Dijkhuizen EH, Wulffraat NM (2015) Early predictors of prognosis in juvenile idiopathic arthritis: a systematic literature review. Ann Rheum Dis 74(11):1996–2005
Adib N, Silman A, Thomson W (2005) Outcome following onset of juvenile idiopathic inflammatory arthritis: I. frequency of different outcomes. Rheumatology (Oxf) 44(8):995–1001
Wallace CA, Huang B, Bandeira M, Ravelli A, Giannini EH (2005) Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum 52(11):3554–3562
Flato B, Lien G, Smerdel A, Vinje O, Dale K, Johnston V et al (2003) Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 14.9 years. J Rheumatol 30(2):386–393
Fantini F, Gerloni V, Gattinara M, Cimaz R, Arnoldi C, Lupi E (2003) Remission in juvenile chronic arthritis: a cohort study of 683 consecutive cases with a mean 10 year followup. J Rheumatol 30(3):579–584
Bertilsson L, Andersson-Gare B, Fasth A, Forsblad-D’elia H (2012) A 5-year prospective population-based study of juvenile chronic arthritis: onset, disease process, and outcome. Scand J Rheumatol 41(5):379–382
Minden K, Kiessling U, Listing J, Niewerth M, Doring E, Meincke J et al (2000) Prognosis of patients with juvenile chronic arthritis and juvenile spondyloarthropathy. J Rheumatol 27(9):2256–2263
Nordal EB, Zak M, Aalto K, Berntson L, Fasth A, Herlin T et al (2012) Validity and predictive ability of the juvenile arthritis disease activity score based on CRP versus ESR in a Nordic population-based setting. Ann Rheum Dis 71(7):1122–1127
Giannini EH, Brewer EJ (1987) Poor correlation between the erythrocyte sedimentation rate and clinical activity in juvenile rheumatoid arthritis. Clin Rheumatol 6(2):197–201
Petty RE, Laxer RM, Lindsley CB, Wedderburn LR. Juvenile Idiopathic Arthritis. Textbook of Pediatric Rheumatology. 7 edn. Elsevier-Saunders, Philadelphia; 2016. 188–204
Kushner I, Samols D, Magrey M. A unifying biologic explanation for “high-sensitivity” C-reactive protein and “low-grade” inflammation. Arthritis Care Res (Hoboken) 2010; 62(4):442–446.
Kushner I, Rzewnicki D, Samols D (2006) What does minor elevation of C-reactive protein signify? Am J Med 119(2):166-e17
Buckley DI, Fu R, Freeman M, Rogers K, Helfand M (2009) C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med 151(7):483–495
Musunuru K, Kral BG, Blumenthal RS, Fuster V, Campbell CY, Gluckman TJ et al (2008) The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med 5(10):621–635
Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW et al (2004) Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350(23):2362–2374
Devaraj S, Singh U, Jialal I (2009) Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol 20(3):182–189
Sennels H, Sorensen S, Ostergaard M, Knudsen L, Hansen M, Skjodt H et al (2008) Circulating levels of osteopontin, osteoprotegerin, total soluble receptor activator of nuclear factor-kappa B ligand, and high-sensitivity C-reactive protein in patients with active rheumatoid arthritis randomized to etanercept alone or in combination with methotrexate. Scand J Rheumatol 37(4):241–247
Hiura K, Iwaki-Egawa S, Kawashima T, Fujisawa S, Takeda T, Komori H et al (2013) The diagnostic utility of matrix metalloproteinase-3 and high-sensitivity C-reactive protein for predicting rheumatoid arthritis in anti-cyclic citrullinated peptide antibody-negative patients with recent-onset undifferentiated arthritis. Rheumatol Int 33(9):2309–2314
Jednacz E, Rutkowska-Sak L (2015) Assessment of the body composition and parameters of the cardiovascular risk in juvenile idiopathic arthritis. Biomed Res Int 2015:619023
Gerss J, Roth J, Holzinger D, Ruperto N, Wittkowski H, Frosch M et al (2012) Phagocyte-specific S100 proteins and high-sensitivity C reactive protein as biomarkers for a risk-adapted treatment to maintain remission in juvenile idiopathic arthritis: a comparative study. Ann Rheum Dis 71(12):1991–1997
De BF, Martini A (1998) Is systemic juvenile rheumatoid arthritis an interleukin 6 mediated disease? J Rheumatol 25(2):203–207
De BF, Martini A (2005) Targeting the interleukin-6 receptor: a new treatment for systemic juvenile idiopathic arthritis? Arthritis Rheum 52(3):687–693
Ravelli A, Martini A (2007) Juvenile idiopathic arthritis. The Lancet 369(9563):767–778
Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. American college of rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011; 63(7):929–36.
Viola S, Felici E, Magni-Manzoni S, Pistorio A, Buoncompagni A, Ruperto N et al (2005) Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheum 52(7):2092–2102
ICSH recommendations for measurement of erythrocyte sedimentation rate (1993) International Council for Standardization in Haematology (Expert Panel on Blood Rheology). J Clin Pathol 46(3):198–203
Fagan TJ (1975) Letter: nomogram for Bayes theorem. N Engl J Med 293(5):257
Oen K, Tucker L, Huber AM, Miettunen P, Scuccimarri R, Campillo S et al (2009) Predictors of early inactive disease in a juvenile idiopathic arthritis cohort: results of a Canadian multicenter, prospective inception cohort study. Arthritis Rheum 61(8):1077–1086
Hyrich KL, Lal SD, Foster HE, Thornton J, Adib N, Baildam E et al (2010) Disease activity and disability in children with juvenile idiopathic arthritis one year following presentation to paediatric rheumatology. Results from the Childhood Arthritis Prospective Study. Rheumatology (Oxf) 49(1):116–122
Esbjornsson AC, Aalto K, Brostrom EW, Fasth A, Herlin T, Nielsen S et al. Ankle arthritis predicts polyarticular disease course and unfavourable outcome in children with juvenile idiopathic arthritis. Clin Exp Rheumatol 2015
Flato B, Hoffmann-Vold AM, Reiff A, Forre O, Lien G, Vinje O (2006) Long-term outcome and prognostic factors in enthesitis-related arthritis: a case-control study. Arthritis Rheum 54(11):3573–3582
Haasnoot AJ, van Tent-Hoeve M, Wulffraat NM, Schalij-Delfos NE, Los LI, Armbrust W et al (2015) Erythrocyte sedimentation rate as baseline predictor for the development of uveitis in children with juvenile idiopathic arthritis. Am J Ophthalmol 159(2):372–377
Pelegrin L, Casaroli-Marano R, Anton J, Garcia de Vicuna MC, Molina-Prat N, Ignacio AJ et al (2014) Predictive value of selected biomarkers, polymorphisms, and clinical features for oligoarticular juvenile idiopathic arthritis-associated uveitis. Ocul Immunol Inflamm 22(3):208–212
Guzman J, Oen K, Huber AM, Watanabe DK, Boire G, Shiff N et al. The risk and nature of flares in juvenile idiopathic arthritis: results from the ReACCh-Out cohort. Ann Rheum Dis 2015
Acknowledgements
This work was supported by grants from the Department of Pediatrics, Naestved hospital, Region Zealand, the Research Department, Region Zealand, The Danish Arthritis foundation, and the Aase and Ejnar Danielsen Foundation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Alberdi-Saugstrup, M., Zak, M., Nielsen, S. et al. High-sensitive CRP as a predictive marker of long-term outcome in juvenile idiopathic arthritis. Rheumatol Int 37, 695–703 (2017). https://doi.org/10.1007/s00296-017-3657-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3657-x