Abstract
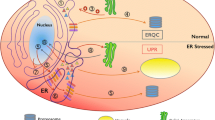
During stress, accumulation of misfolded proteins in the endoplasmic reticulum (ER) triggers activation of the adaptive mechanisms that restore protein homeostasis. One mechanism that eukaryotic cells use to respond to ER stress is through activation of the unfolded protein response (UPR) signaling pathway, which initiates degradation of misfolded proteins and leads to inhibition of translation and increased expression of chaperones and oxidative folding components that enhance ER protein folding capacity. However, the mechanisms of adaptation to ER stress are not limited to the UPR. Using yeast Saccharomyces cerevisiae, we recently discovered that the protein folding burden in the ER can be alleviated in a UPR-independent manner through duplication of whole chromosomes containing ER stress-protective genes. Here we discuss our findings and their implication to our understanding of the mechanisms by which cells respond to protein misfolding in the ER.

Similar content being viewed by others
References
Barnes G, Hansen WJ, Holcomb CL, Rine J (1984) Asparagine-linked glycosylation in Saccharomyces cerevisiae: genetic analysis of an early step. Mol Cell Biol 4(11):2381–2388
Beaupere C et al (2018) Genetic screen identifies adaptive aneuploidy as a key mediator of ER stress resistance in yeast. Proc Natl Acad Sci USA 115(38):9586–9591
Bretthauer RK (2009) Structure, expression, and regulation of UDP-GlcNAc: dolichol phosphate GlcNAc-1-phosphate transferase (DPAGT1). Curr Drug Targets 10(6):477–482
Chawla A, Chakrabarti S, Ghosh G, Niwa M (2011) Attenuation of yeast UPR is essential for survival and is mediated by IRE1 kinase. J Cell Biol 193(1):41–50
Chen G, Bradford WD, Seidel CW, Li R (2012) Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature 482(7384):246–250
Defenouillere Q, Fromont-Racine M (2017) The ribosome-bound quality control complex: from aberrant peptide clearance to proteostasis maintenance. Curr Genet 63(6):997–1005
Denzel MS, et al (2014) Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell 156(6):1167–1178
Dephoure N et al (2014) Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. eLife 3:e03023
Finley D, Chen X, Walters KJ (2016) Gates, channels, and switches: elements of the proteasome machine. Trends Biochem Sci 41(1):77–93
Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P (2013) Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol 5(3):a013169
Giaever G et al (1999) Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat Genet 21(3):278–283
Gottschling DE, Nystrom T (2017) The upsides and downsides of organelle interconnectivity. Cell 169(1):24–34
Groll M et al (1999) The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc Natl Acad Sci USA 96(20):10976–10983
Helenius A, Aebi M (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem 73:1019–1049
Karagoz GE et al (2017) An unfolded protein-induced conformational switch activates mammalian IRE1. eLife 6:e30700
Kaya A et al (2015) Adaptive aneuploidy protects against thiol peroxidase deficiency by increasing respiration via key mitochondrial proteins. Proc Natl Acad Sci USA 112(34):10685–10690
Koga H, Kaushik S, Cuervo AM (2011) Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev 10(2):205–215
Koike N, Hatano Y, Ushimaru T (2018) Heat shock transcriptional factor mediates mitochondrial unfolded protein response. Curr Genet 64(4):907–917
Labunskyy VM et al (2014) Lifespan extension conferred by endoplasmic reticulum secretory pathway deficiency requires induction of the unfolded protein response. PLoS Genet 10(1):e1004019
Lennon K, Bird A, Chen YF, Pretel R, Kukuruzinska MA (1997) The dual role of mRNA half-lives in the expression of the yeast ALG7 gene. Mol Cell Biochem 169(1–2):95–106
Livneh I, Cohen-Kaplan V, Cohen-Rosenzweig C, Avni N, Ciechanover A (2016) The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Res 26(8):869–885
Oromendia AB, Dodgson SE, Amon A (2012) Aneuploidy causes proteotoxic stress in yeast. Genes Dev 26(24):2696–2708
Pavelka N et al (2010) Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 468(7321):321–325
Pereira T et al (2018) Quantitative operating principles of yeast metabolism during adaptation to heat stress. Cell Rep 22(9):2421–2430
Pincus D et al (2010) BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol 8(7):e1000415
Selmecki A, Forche A, Berman J (2006) Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313(5785):367–370
Stauffer B, Powers T (2017) Target of rapamycin signaling mediates vacuolar fragmentation. Curr Genet 63(1):35–42
Sunshine AB et al (2015) The fitness consequences of aneuploidy are driven by condition-dependent gene effects. PLoS Biol 13(5):e1002155
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13(3):184–190
Torres EM et al (2007) Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317(5840):916–924
Travers KJ et al (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101(3):249–258
Van Dalfsen KM et al (2018) Global proteome remodeling during ER stress involves Hac1-driven expression of long undecoded transcript isoforms. Dev Cell 46(2):219–235 (e218)
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086
Weimer S et al (2014) d-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat Commun 5:3563
Yona AH et al (2012) Chromosomal duplication is a transient evolutionary solution to stress. Proc Natl Acad Sci USA 109(51):21010–21015
Yoshida H (2007) ER stress and diseases. FEBS J 274(3):630–658
Zhu J, Tsai HJ, Gordon MR, Li R (2018) Cellular stress associated with aneuploidy. Dev Cell 44(4):420–431
Acknowledgements
This work was supported by the National Institutes of Health Grant AG054566 (to VML). This research was conducted while VML was an AFAR Research Grant recipient from the American Federation for Aging Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Beaupere, C., Labunskyy, V.M. (Un)folding mechanisms of adaptation to ER stress: lessons from aneuploidy. Curr Genet 65, 467–471 (2019). https://doi.org/10.1007/s00294-018-0914-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-018-0914-9