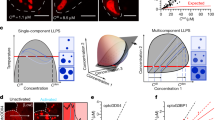
Abstract
The bacterial cytoplasm, once thought to be a relatively undifferentiated reaction medium, has now been recognized to have extensive microstructure. This microstructure includes bacterial microcompartments, inclusion bodies, granules, and even some membrane-bound vesicles. Several recent papers suggest that bacteria may also organize their cytoplasm using an additional mechanism: phase-separated membraneless organelles, a strategy commonly used by eukaryotes. Phase-separated membraneless organelles such as Cajal bodies, the nucleolus, and stress granules allow proteins to become concentrated in sub-compartments of eukaryotic cells without being surrounded by a barrier to diffusion. In this review, we summarize the known structural organization of the bacterial cytoplasm and discuss the recent evidence that phase-separated membraneless organelles might also play a role in bacterial systems. We specifically focus on bacterial ribonucleoprotein complexes and two different protein components of the bacterial nucleoid that may have the ability to form subcellular partitions within bacteria cells.

Similar content being viewed by others
References
Azam TA, Ishihama A (1999) Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity DNA binding affinity. J Biol Chem 274:33105–33113
Azam TA, Iwata A, Nishimura A, Ueda S, Ishihama A (1999) Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol 181:6361–6370
Banani SF, Lee HO, Hyman AA, Rosen MK (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18:285–298. https://doi.org/10.1038/nrm.2017.7
Bernhardt TG, de Boer PA (2005) SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol cell 18:555–564. https://doi.org/10.1016/j.molcel.2005.04.012
Boeynaems S et al (2018) Protein phase separation: a new phase in cell biology. Trends Cell Biol 28:420–435. https://doi.org/10.1016/j.tcb.2018.02.004
Brangwynne CP et al (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324:1729–1732. https://doi.org/10.1126/science.1172046
Cornejo E, Abreu N, Komeili A (2014) Compartmentalization and organelle formation in bacteria. Curr Opin Cell Biol 26:132–138. https://doi.org/10.1016/j.ceb.2013.12.007
Cox B, Tuite M (2018) The life of [PSI]. Curr Genet 64:1–8. https://doi.org/10.1007/s00294-017-0714-7
Enenkel C (2018) The paradox of proteasome granules. Curr Genet 64:137–140. https://doi.org/10.1007/s00294-017-0739-y
Feric M et al (2016) Coexisting liquid phases underlie. Nucleolar Subcompart Cell 165:1686–1697. https://doi.org/10.1016/j.cell.2016.04.047
Frenkiel-Krispin D et al (2001) Regulated phase transitions of bacterial chromatin: a non-enzymatic pathway for generic DNA protection. Embo J 20:1184–1191. https://doi.org/10.1093/emboj/20.5.1184
Gomes E, Shorter J (2018) The molecular language of membraneless organelles. J Biol Chem. https://doi.org/10.1074/jbc.TM118.001192
Hansma HG (2017) Better than membranes at the origin of life? Life. https://doi.org/10.3390/life7020028
Janissen R et al (2018) Global DNA compaction in stationary-phase bacteria does not affect transcription. Cell 174:1188–1199 e1114. https://doi.org/10.1016/j.cell.2018.06.049
Jun S, Mulder B (2006) Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc Natl Acad Sci USA 103:12388–12393. https://doi.org/10.1073/pnas.0605305103
Karas VO, Westerlaken I, Meyer AS (2015) The DNA-binding protein from starved cells (Dps) utilizes dual functions to defend cells against multiple stresses. J Bacteriol 197:3206–3215. https://doi.org/10.1128/JB.00475-15
Kerfeld CA, Aussignargues C, Zarzycki J, Cai F, Sutter M (2018) Bacterial microcompartments. Nat Rev Microbiol 16:277–290. https://doi.org/10.1038/nrmicro.2018.10
Kim J, Yoshimura SH, Hizume K, Ohniwa RL, Ishihama A, Takeyasu K (2004) Fundamental structural units of the Escherichia coli nucleoid revealed by atomic force microscopy. Nucleic Acids Res 32:1982–1992. https://doi.org/10.1093/nar/gkh512
Langdon EM, Gladfelter AS (2018) A new lens for RNA localization liquid–liquid phase separation. Annu Rev Microbiol 72:255–271. https://doi.org/10.1146/annurev-micro-090817-062814
Lutkenhaus J (2007) Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem 76:539–562. https://doi.org/10.1146/annurev.biochem.75.103004.142652
Molliex A et al (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163:123–133. https://doi.org/10.1016/j.cell.2015.09.015
Monterroso B, Zorrilla S, Sobrinos-Sanguino M, Keating CD, Rivas G (2016) Microenvironments created by liquid–liquid phase transition control the dynamic distribution of bacterial division FtsZ protein. Sci Rep 6:35140. https://doi.org/10.1038/srep35140
Monterroso B, Zorrilla S, Sobrinos-Sanguino M, Robles-Ramos MA, López-Álvarez M, Keating CD, Rivas G (2018) Bacterial division FtsZ forms liquid condensates with nucleoid-associated Z-ring inhibitor. SlmA bioRxiv https://doi.org/10.1101/264192
Nevo-Dinur K, Nussbaum-Shochat A, Ben-Yehuda S, Amster-Choder O (2011) Translation-independent localization of mRNA in E. coli. Science 331:1081–1084. https://doi.org/10.1126/science.1195691
Nott TJ et al (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol cell 57:936–947. https://doi.org/10.1016/j.molcel.2015.01.013
Odijk T (1998) Osmotic compaction of supercoiled DNA into a bacterial nucleoid. Biophys Chem 73:23–29
Pederson T (2011) The nucleolus. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a000638
Pelletier J et al (2012) Physical manipulation of the Escherichia coli chromosome reveals its soft nature. Proc Natl Acad Sci USA 109:E2649–E2656. https://doi.org/10.1073/pnas.1208689109
Racki LR, Tocheva EI, Dieterle MG, Sullivan MC, Jensen GJ, Newman DK (2017) Polyphosphate granule biogenesis is temporally and functionally tied to cell cycle exit during starvation in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 114:E2440–E2449. https://doi.org/10.1073/pnas.1615575114
Rinas U, Garcia-Fruitos E, Corchero JL, Vazquez E, Seras-Franzoso J, Villaverde A (2017) Bacterial inclusion bodies: discovering their better half. Trends Biochem Sci 42:726–737. https://doi.org/10.1016/j.tibs.2017.01.005
Russell JH, Keiler KC (2009) Subcellular localization of a bacterial regulatory RNA. Proc Natl Acad Sci USA 106:16405–16409. https://doi.org/10.1073/pnas.0904904106
Ryzhova TA et al (2018) Screening for amyloid proteins in the yeast proteome. Curr Genet 64:469–478. https://doi.org/10.1007/s00294-017-0759-7
Shapiro L, McAdams HH, Losick R (2009) Why and how bacteria. localize proteins. Science 326:1225–1228. https://doi.org/10.1126/science.1175685
Simpson-Lavy K, Kupiec M (2018) A reversible liquid drop aggregation controls glucose response in yeast. Curr Genet 64:785–788. https://doi.org/10.1007/s00294-018-0805-0
Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, Karpen GH (2017) Phase separation drives heterochromatin domain formation. Nature 547:241–245. https://doi.org/10.1038/nature22989
Sutter M et al (2008) Structural basis of enzyme encapsulation into a bacterial nanocompartment. Nat Struct Mol Biol 15:939–947. https://doi.org/10.1038/nsmb.1473
Valkenburg JA, Woldringh CL (1984) Phase separation between nucleoid and cytoplasm in Escherichia coli as defined by immersive refractometry. J Bacteriol 160:1151–1157
Vtyurina NN, Dulin D, Docter MW, Meyer AS, Dekker NH, Abbondanzieri EA (2016) Hysteresis in DNA compaction by Dps is described by an Ising model. Proc Natl Acad Sci USA 113:4982–4987. https://doi.org/10.1073/pnas.1521241113
Wang W, Li GW, Chen C, Xie XS, Zhuang X (2011) Chromosome organization by a nucleoid-associated protein in live bacteria. Science 333:1445–1449. https://doi.org/10.1126/science.1204697
Wang X, Montero Llopis P, Rudner DZ (2013) Organization and segregation of bacterial chromosomes. Nat Rev Genet 14:191–203. https://doi.org/10.1038/nrg3375
Wisniewski BT, Sharma J, Legan ER, Paulson E, Merrill SJ, Manogaran AL (2018) Toxicity and infectivity: insights from de novo prion formation. Curr Genet 64:117–123. https://doi.org/10.1007/s00294-017-0736-1
Wolf SG, Frenkiel D, Arad T, Finkel SE, Kolter R, Minsky A (1999) DNA protection by stress-induced biocrystallization. Nature 400:83–85
Yuan AH, Hochschild A (2017) A bacterial global regulator forms a prion. Science 355:198–201. https://doi.org/10.1126/science.aai7776
Zhang P, Khursigara CM, Hartnell LM, Subramaniam S (2007) Direct visualization of Escherichia coli chemotaxis receptor arrays using cryo-electron microscopy. Proc Natl Acad Sci USA 104:3777–3781. https://doi.org/10.1073/pnas.0610106104
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbondanzieri, E.A., Meyer, A.S. More than just a phase: the search for membraneless organelles in the bacterial cytoplasm. Curr Genet 65, 691–694 (2019). https://doi.org/10.1007/s00294-018-00927-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-018-00927-x