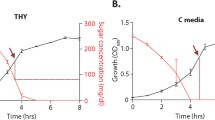
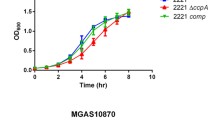
Abstract
A growing body of evidence points to CodY, a global regulator in Gram-positive bacteria, as a critical link between microbial physiology and pathogenesis in diverse environments. Recent studies uncovering graded regulation of CodY gene targets reflect the true nature of this transcription factor controlled by ligands and reveal nutrient availability as a potentially critical factor in modulating pathogenesis. This review will serve to update the status of the field and raise new questions to be answered.



Similar content being viewed by others
References
Ababneh QO, Herman JK (2015) CodY regulates SigD levels and activity by binding to three sites in the fla/che operon. J Bacteriol 197:2999–3006
Alonzo Fr, Torres V (2014) The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev 78:199–230
Balaban N, Novick RP (1995) Autocrine regulation of toxin synthesis by Staphylococcus aureus. Proc Natl Acad Sci USA 92:1619–1623
Balasubramanian D et al (2016) Staphylococcus aureus coordinates leukocidin expression and pathogenesis by sensing metabolic fluxes via RpiRc. MBio 7(3):e00818-e00816
Barbieri G, Albertini AM, Ferrari E, Sonenshein AL, Belitsky BR (2016) Interplay of CodY and ScoC in the regulation of major extracellular protease genes of Bacillus subtilis. J Bacteriol 198:907–920
Belitsky BR (2011) Indirect repression by Bacillus subtilis CodY via displacement of the activator of the proline utilization operon. J Mol Biol 413:321–336
Belitsky BR, Sonenshein AL (2008) Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis. J Bacteriol 190:1224–1236
Belitsky BR, Sonenshein AL (2011a) Contributions of multiple binding sites and effector-independent binding to CodY-mediated regulation in Bacillus subtilis. J Bacteriol 193:473–484
Belitsky BR, Sonenshein AL (2011b) Roadblock repression of transcription by Bacillus subtilis CodY. J Mol Biol 411:729–743
Belitsky BR, Sonenshein AL (2013) Genome-wide identification of Bacillus subtilis CodY-binding sites at single-nucleotide resolution. Proc Natl Acad Sci USA 110:7026–7031
Belitsky BR, Barbieri G, Albertini AM, Ferrari E, Strauch MA, Sonenshein AL (2015a) Interactive regulation by the Bacillus subtilis global regulators CodY and ScoC. Mol Microbiol 97:698–716
Belitsky BR, Brinsmade SR, Sonenshein AL (2015b) Intermediate levels of Bacillus subtilis CodY activity are required for derepression of the branched-chain amino acid permease, BraB. PLoS Genet 11:e1005600
Berube BJ, Bubeck Wardenburg J (2013) Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166
Böhm ME, Krey VM, Jeßberger N, Frenzel E, Scherer S (2016) Comparative bioinformatics and experimental analysis of the intergenic regulatory regions of Bacillus cereus hbl and nhe enterotoxin operons and the impact of CodY on virulence heterogeneity. Front Microbiol 7:768
Bouillaut L, Dubois T, Sonenshein AL, Dupuy B (2015) Integration of metabolism and virulence in Clostridium difficile. Res Microbiol 166:375–383
Brinsmade SR, Sonenshein AL (2011) Dissecting complex metabolic integration provides direct genetic evidence for CodY activation by guanine nucleotides. J Bacteriol 193:5637–5648
Brinsmade SR, Kleijn RJ, Sauer U, Sonenshein AL (2010) Regulation of CodY activity through modulation of intracellular branched-chain amino acid pools. J Bacteriol 192:6357–6368
Brinsmade S, Alexander E, Livny J, Stettner A, Segrè D, Rhee K, Sonenshein A (2014) Hierarchical expression of genes controlled by the Bacillus subtilis global regulatory protein CodY. Proc Natl Acad Sci USA 111:8227–8232
Bubeck Wardenburg J, Patel R, Schneewind O (2007) Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75:1040–1044
Casewell M, Hill R (1986) The carrier state: methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 18(Suppl A):1–12
Château A, van Schaik W, Six A, Aucher W, Fouet A (2011) CodY regulation is required for full virulence and heme iron acquisition in Bacillus anthracis. FASEB J 25:4445–4456
Château A et al (2013) Identification of CodY targets in Bacillus anthracis by genome-wide in vitro binding analysis. J Bacteriol 195:1204–1213
Cheng A, Kim H, Burts M, Krausz T, Schneewind O, Missiakas D (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J 23:3393–3404
Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O (2010) Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog 6:e1001036
Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS (2010) ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev 74:171–199
de Haas C et al (2004) Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199:687–695
den Hengst CD, van Hijum SAFT, Geurts JMW, Nauta A, Kok J, Kuipers OP (2005) The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J Biol Chem 280:34332–34342
Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL (2007) Repression of Clostridium difficile toxin gene expression by CodY. Mol Microbiol 66:206–219
Dineen SS, McBride SM, Sonenshein AL (2010) Integration of metabolism and virulence by Clostridium difficile CodY. J Bacteriol 192:5350–5362
DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, Torres VJ (2013) Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun 81:1830–1841
Durham D (1970) Distribution of free amino acids in human intraocular fluids. Trans Am Ophthalmol Soc 68:462–500
Ehling-Schulz M, Frenzel E, Gohar M (2015) Food-bacteria interplay: pathometabolism of emetic Bacillus cereus. Front Microbiol 6:704
Feng L et al (2016) The CodY regulator is essential for virulence in Streptococcus suis serotype 2. Sci Rep 6:21241
Flannagan RS, Heit B, Heinrichs DE (2015) Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 4:826–868
Flannagan RS, Heit B, Heinrichs DE (2016) Intracellular replication of Staphylococcus aureus in mature phagolysosomes in macrophages precedes host cell death, and bacterial escape and dissemination. Cell Microbiol 18:514–535
Fraunholz M, Sinha B (2012) Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol 2:43
Gaca AO, Colomer-Winter C, Lemos JA (2015) Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J Bacteriol 197:1146–1156
Gaupp R et al (2016) RpiRc is a pleiotropic effector of virulence determinant synthesis and attenuates pathogenicity in Staphylococcus aureus. Infect Immun 84:2031–2041
Geiger T, Wolz C (2014) Intersection of the stringent response and the CodY regulon in low GC Gram-positive bacteria. Int J Med Microbiol 304:150–155
Geiger T, Goerke C, Mainiero M, Kraus D, Wolz C (2008) The virulence regulator Sae of Staphylococcus aureus: promoter activities and response to phagocytosis-related signals. J Bacteriol 190:3419–3428
Geiger T et al (2010) Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect Immun 78:1873–1883
Geiger T et al (2012) The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016
Geisinger E, Adhikari RP, Jin R, Ross HF, Novick RP (2006) Inhibition of rot translation by RNAIII, a key feature of agr function. Mol Microbiol 61:1038–1048
George SE, Nguyen T, Geiger T, Weidenmaier C, Lee JC, Liese J, Wolz C (2015) Phenotypic heterogeneity and temporal expression of the capsular polysaccharide in Staphylococcus aureus. Mol Microbiol 98:1073–1088
Gillaspy A, Hickmon S, Skinner R, Thomas J, Nelson C, Smeltzer M (1995) Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun 63:3373–3380
Gopalani M, Dhiman A, Rahi A, Bhatnagar R (2016) Overexpression of the pleiotropic regulator CodY decreases sporulation, attachment and pellicle formation in Bacillus anthracis. Biochem Biophys Res Commun 469:672–678
Greene C, McDevitt D, Francois P, Vaudaux P, Lew D, Foster T (1995) Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol 17:1143–1152
Grosser MR, Weiss A, Shaw LN, Richardson AR (2016) Regulatory requirements for Staphylococcus aureus nitric oxide resistance. J Bacteriol 198:2043–2055
Grundy FJ, Henkin TM (1993) tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell 74:475–482
Grundy FJ, Henkin TM (1998) The S box regulon: a new global transcription termination control system for methionine and cysteine biosynthesis genes in gram-positive bacteria. Mol Microbiol 30:737–749
Guédon E, Serror P, Ehrlich SD, Renault P, Delorme C (2001) Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol 40:1227–1239
Han AR et al (2016) The structure of the pleiotropic transcription regulator CodY provides insight into its GTP-sensing mechanism. Nucleic Acids Res. doi:10.1093/nar/gkw775
Handlogten ME, Hong SP, Westhoff CM, Weiner ID (2005) Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289:F347–F358
Hartleib J et al (2000) Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood 96:2149–2156
Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK (2014) Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie 96:121–129
Huang SC, Burne RA, Chen YY (2014) The pH-dependent expression of the urease operon in Streptococcus salivarius is mediated by CodY. Appl Environ Microbiol 80:5386–5393
Ibberson C, Jones C, Singh S, Wise M, Hart M, Zurawski D, Horswill A (2014) Staphylococcus aureus hyaluronidase is a CodY-regulated virulence factor. Infect Immun 82:4253–4264
Jeong DW et al (2012) The auxiliary protein complex SaePQ activates the phosphatase activity of sensor kinase SaeS in the SaeRS two-component system of Staphylococcus aureus. Mol Microbiol 86:331–348
Joshi G, Spontak J, Klapper D, Richardson A (2011) Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol Microbiol 82:9–20
Kaiser J, Omer S, Sheldon J, Welch I, Heinrichs D (2015) Role of BrnQ1 and BrnQ2 in branched-chain amino acid transport and virulence in Staphylococcus aureus. Infect Immun 83:1019–1029
Kaiser JC, Sen S, Sinha A, Wilkinson BJ, Heinrichs DE (2016) The role of two branched-chain amino acid transporters in Staphylococcus aureus growth, membrane fatty acid composition, and virulence. Mol Microbiol. doi:10.1111/mmi.13495
Kim SK, Jung KH, Chai YG (2016a) Changes in Bacillus anthracis CodY regulation under host-specific environmental factor deprived conditions. BMC Genom 17:645
Kim SK, Jung KH, Yoon SN, Kim YK, Chai YG (2016b) Late-Exponential Gene Expression in CodY-Deficient Bacillus anthracis in a Host-Like Environment. Curr Microbiol 73:714–720
Kovács ÁT (2016) The global regulator CodY is required for the fitness of Bacillus cereus in various laboratory media and certain beverages. FEMS Microbiol Lett 363:fnw126
Krismer B et al (2014) Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10:e1003862
Kumar N, David M, Boyle-Vavra S, Sieth J, Daum R (2015) High Staphylococcus aureus colonization prevalence among patients with skin and soft tissue infections and controls in an urban emergency department. J Clin Microbiol 53:810–815
Lee B et al (2013) The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect 19:528–536
Levdikov VM, Blagova E, Colledge VL, Lebedev AA, Williamson DC, Sonenshein AL, Wilkinson AJ (2009) Structural rearrangement accompanying ligand binding in the GAF domain of CodY from Bacillus subtilis. J Mol Biol 390:1007–1018
Li D, Cheung A (2008) Repression of hla by rot is dependent on sae in Staphylococcus aureus. Infect Immun 76:1068–1075
Li J, Freedman JC, McClane BA (2015) NanI Sialidase, CcpA, and CodY work together to regulate epsilon toxin production by Clostridium perfringens type D strain CN3718. J Bacteriol 197:3339–3353
Lobel L, Herskovits AA (2016) Systems level analyses reveal multiple regulatory activities of CodY controlling metabolism, motility and virulence in Listeria monocytogenes. PLoS Genet 12:e1005870
Lobel L, Sigal N, Borovok I, Ruppin E, Herskovits AA (2012) Integrative genomic analysis identifies isoleucine and CodY as regulators of Listeria monocytogenes virulence. PLoS Genet 8:e1002887
Lobel L, Sigal N, Borovok I, Belitsky BR, Sonenshein AL, Herskovits AA (2015) The metabolic regulator CodY links Listeria monocytogenes metabolism to virulence by directly activating the virulence regulatory gene prfA. Mol Microbiol 95:624–644
Lowy F (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532
Luong T, Sau S, Gomez M, Lee JC, Lee CY (2002) Regulation of Staphylococcus aureus capsular polysaccharide expression by agr and sarA. Infect Immun 70:444–450
Mainiero M, Goerke C, Geiger T, Gonser C, Herbert S, Wolz C (2010) Differential target gene activation by the Staphylococcus aureus two-component system saeRS. J Bacteriol 192:613–623
Majerczyk CD, Sadykov MR, Luong TT, Lee C, Somerville GA, Sonenshein AL (2008) Staphylococcus aureus CodY negatively regulates virulence gene expression. J Bacteriol 190:2257–2265
Majerczyk CD et al (2010) Direct targets of CodY in Staphylococcus aureus. J Bacteriol 192:2861–2877
Molle V, Nakaura Y, Shivers RP, Yamaguchi H, Losick R, Fujita Y, Sonenshein AL (2003) Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J Bacteriol 185:1911–1922
Montgomery CP, Boyle-Vavra S, Roux A, Ebine K, Sonenshein AL, Daum RS (2012) CodY deletion enhances in vivo virulence of community-associated methicillin-resistant Staphylococcus aureus USA300 Infect Immun 80:2382–2389
Münzenmayer L, Geiger T, Daiber E, Schulte B, Autenrieth SE, Fraunholz M, Wolz C (2016) Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell Microbiol 18:1172–1183
Nakatsukasa M et al (2011) Amino Acid profiles in human tear fluids analyzed by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. Am J Ophthalmol 151:799–808
Nasset ES, Heald FP, Calloway DH, Margen S, Schneeman P (1979) Amino acids in human blood plasma after single meals of meat, oil, sucrose and whiskey. J Nutr 109:621–630
Nawrocki KL, Edwards AN, Daou N, Bouillaut L, McBride SM (2016) CodY-Dependent Regulation of Sporulation in Clostridium difficile. J Bacteriol 198:2113–2130
Noble W, Valkenburg H, Wolters C (1967) Carriage of Staphylococcus aureus in random samples of a normal population. J Hyg (Lond) 65:567–573
Novick R, Jiang D (2003) The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 149:2709–2717
Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S (1993) Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12:3967–3975
Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM (2010) SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis 201:241–254
Olson M et al (2013) Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infect Immun 81:1316–1324
Otto M (2013) Community-associated MRSA: what makes them special? Int J Med Microbiol 303:324–330
Petranovic D, Guédon E, Sperandio B, Delorme C, Ehrlich D, Renault P (2004) Intracellular effectors regulating the activity of the Lactococcus lactis CodY pleiotropic transcription regulator. Mol Microbiol 53:613–621
Pohl K et al (2009) CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J Bacteriol 191:2953–2963
Potrykus K, Cashel M (2008) (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51
Ratnayake-Lecamwasam M, Serror P, Wong K-W, Sonenshein AL (2001) Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev 15:1093–1103
Reiss S et al (2012) Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob Agents Chemother 56:787–804
Richardson AR, Somerville GA, Sonenshein AL (2015) Regulating the intersection of metabolism and pathogenesis in Gram-positive bacteria. Microbiol Spectr. doi:10.1128/microbiolspec.MBP-0004-2014
Sadaka A, Palmer K, Suzuki T, Gilmore MS (2014) In vitro and in vivo models of Staphylococcus aureus endophthalmitis implicate specific nutrients in ocular Infection. PLoS One 9:e110872
Salgado-Pabón W et al (2013) Superantigens are critical for Staphylococcus aureus infective endocarditis, sepsis, and acute kidney injury. MBio 4:e00494–e00513
Schoenfelder SM, Marincola G, Geiger T, Goerke C, Wolz C, Ziebuhr W (2013) Methionine biosynthesis in Staphylococcus aureus is tightly controlled by a hierarchical network involving an initiator tRNA-specific T-box riboswitch. PLoS Pathog 9:e1003606
Serganov A et al (2004) Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem Biol 11:1729–1741
Shivers RP, Sonenshein AL (2004) Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol Microbiol 53:599–611
Shivers RP, Dineen SS, Sonenshein AL (2006) Positive regulation of Bacillus subtilis ackA by CodY and CcpA: establishing a potential hierarchy in carbon flow. Mol Microbiol 62:811–822
Slack FJ, Serror P, Joyce E, Sonenshein AL (1995) A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol Microbiol 15:689–702
Slamti L, Lemy C, Henry C, Guillot A, Huillet E, Lereclus D (2016) CodY regulates the activity of the virulence quorum sensor PlcR by controlling the import of the signaling peptide PapR in Bacillus thuringiensis. Front Microbiol 6:1501
Somerville GA, Proctor RA (2009) At the crossroads of bacterial metabolism and virulence factor synthesis in staphylococci. Microbiol Mol Biol Rev 73:233–248
Sonenshein AL (2005) CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol 8:203–207
Stenz L, Francois P, Whiteson K, Wolz C, Linder P, Schrenzel J (2011) The CodY pleiotropic repressor controls virulence in Gram-positive pathogens. FEMS Immunol Med Microbiol 62:123–139. doi:10.1111/j.1574-695X.2011.00812.x
Sun F, Li C, Jeong D, Sohn C, He C, Bae T (2010) In the Staphylococcus aureus two-component system sae, the response regulator SaeR binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. J Bacteriol 192:2111–2127
Thurlow LR, Joshi GS, Richardson AR (2012) Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). FEMS Immunol Med Microbiol 65:5–22
Thurlow L, Joshi G, Clark J, Spontak J, Neely C, Maile R, Richardson A (2013) Functional modularity of the arginine catabolic mobile element contributes to the success of USA300 methicillin-resistant Staphylococcus aureus. Cell Host Microbe 13:100–107
van Schaik W, Château A, Dillies MA, Coppée JY, Sonenshein AL, Fouet A (2009) The global regulator CodY regulates toxin gene expression in Bacillus anthracis and is required for full virulence. Infect Immun 77:4437–4445
Vandenesch F et al (2003) Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton–Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9:978–984
Villapakkam AC, Handke LD, Belitsky BR, Levdikov VM, Wilkinson AJ, Sonenshein AL (2009) Genetic and biochemical analysis of the interaction of Bacillus subtilis CodY with branched-chain amino acids. J Bacteriol 191:6865–6876
Waters NR, Samuels DJ, Behera RK, Livny J, Rhee KY, Sadykov MR, Brinsmade SR (2016) A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Mol Microbiol 101:495–514
Acknowledgments
I thank Linc Sonenshein and Boris Belitsky for critical comments on the manuscript. Research in the Brinsmade lab is supported by an NIH Pathway to Independence Award (GM099893) and startup funds from Georgetown University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Brinsmade, S.R. CodY, a master integrator of metabolism and virulence in Gram-positive bacteria. Curr Genet 63, 417–425 (2017). https://doi.org/10.1007/s00294-016-0656-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-016-0656-5