Abstract
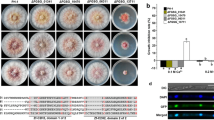
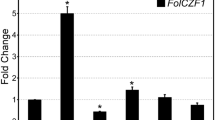
Fusarium verticillioides is both an endophyte and a pathogen of maize and is a health threat in many areas of the world because it can contaminate maize with fumonisins, a toxic secondary metabolite. We identified eight putative chitin synthase (CHS) genes in F. verticillioides genomic sequence, and phylogenetic evidence shows that they group into seven established CHS gene classes. We targeted two CHSs (CHS5 and CHS7) for deletion analysis and found that both are required for normal hyphal growth and maximal disease of maize seedlings and ears. CHS5 and CHS7 encode a putative class V and class VII fungal chitin synthase, respectively; they are located adjacent to each other and are divergently transcribed. Fluorescent microscopy found that both CHS deficient strains produce balloon-shaped hyphae, while growth assays indicated that they were more sensitive to cell wall stressing compounds (e.g., the antifungal compound Nikkomycin Z) than wild type. Pathogenicity assays on maize seedlings and ears indicated that both strains were significantly reduced in their ability to cause disease. Our results demonstrate that both CHS5 and CHS7 are necessary for proper hyphal growth and pathogenicity of F. verticillioides on maize.






Similar content being viewed by others
References
Bacon CW, Hinton DM (1996) Symptomless endophytic colonization of maize by Fusarium moniliforme. Can J Bot 74:1195–1202
Bacon CW, Glenn AE, Yates IE (2008) Fusarium verticillioides: managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev 27:411–446
Bartnicki-Garcia S, Lippman E (1969) Fungal morphogenesis: cell wall construction in Mucor rouxii. Science 165:302–304
Brown DW, McCormick SP, Alexander NJ, Proctor RH, Desjardins AE (2002) Inactivation of a cytochrome P-450 is a determinant of trichothecene diversity in Fusarium species. Fungal Genet Biol 36:224–233
Bulawa CE (1993) Genetics and molecular biology of chitin synthesis in fungi. Annu Rev Microbiol 47:505–534
Butchko RAE, Plattner RD, Proctor RH (2003) FUM13 encodes a short dehydrogenase/reductase required for C-3 carbonyl reduction during fumonisin biosynthesis in Gibberella moniliformis. J Agric Food Chem 51:3000–3006
Cabib E, Shaw JA, Mol PC, Bowers B, Choi WJ (1996) Chitin biosynthesis and morphogenetic processes. In: Brambl R, Marzluf GA (eds) The Mycota. Biochemistry and molecular biology, vol 3. Springer, Berlin, pp 243–276
Capellini RA, Peterson JL (1965) Macroconidium formation in submerged cultures by a non-sporulating strain of Gibberella zeae. Mycologia 57:962–966
Choquer M, Boccara M, Goncalves IR, Soulié MC, Vidal-Cros A (2004) Survey of the Botrytis cinerea chitin synthase multigenic family through the analysis of six euascomycetes genomes. Eur J Biochem 271:2153–2164
Cid VA, Duran A, Del Rey FD, Synder MP, Nombela C, Sanchez M (1995) Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol Rev 59:345–386
Cohen E (1993) Chitin synthesis and degradation as targets for pesticide action. Arch Insect Biochem Physiol 22:245–261
Debono M, Gordee RS (1994) Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol 48:471–497
Desjardins AE, Busman M, Manandhar G, Jarosz AM, Manandhar HK, Proctor RH (2008) Gibberella ear rot of maize (Zea mays) in Nepal: distribution of the mycotoxins nivalenol and deoxynivalenol in naturally and experimentally infected maize. J Agric Food Chem 56:5428–5436
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Glenn AE, Zitomer NC, Zimeri AM, Williams LD, Riley RT, Proctor RH (2008) Transformation-mediated complementation of a Fum gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol Plant Microbe Interact 21:87–97
Howard PC, Eppley RM, Stack ME, Warbritton A, Voss KA, Lorentzen RJ, Kovach RM, Bucci TJ (2001) Fumonisin B1 carcinogenicity is a two-year feeding study using F344 rats and B6C3F1 mice. Environ Health Perspect 109:277–282
Kim J, Lee H, Lee J, Kim KW, Yun S, Shim W, Lee Y (2009) Gibberella zeae chitin synthase genes, GzCHS5 and GzCHS7, are required for hyphal growth, perithecia formation, and pathogenicity. Curr Genet 55:449–549
Lee JI, Yu YM, Rho YM, Park BC, Choi JH, Park HM, Maeng PJ (2005) Differential expression of the chsE gene encoding a chitin synthase of Aspergillus nidulans in response to developmental status and growth conditions. FEMS Microbiol Lett 249:121–129
Leslie JF, Plattner RD, Desjardins AE, Klittich CJR (1992) Fumonisin B1 production by strains from different mating populations of Gibberella fujikuroi (Fusarium section Liseola). Mycotoxicology 82:341–345
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Madrid MP, Di Pietro A, Roncero MIG (2003) Class V chitin synthase determines pathogenesis in the vascular wilt fungus Fusarium oxysporum and mediates resistance to plant defense compounds. Mol Microbiol 47:257–266
Mandel MA, Galgiani JN, Kroken S, Orbach MJ (2006) Coccidioides posadasii contains single chitin synthase genes corresponding to classes I to VII. Fungal Gen Biol 43:775–788
Marek ET, Schardl CL, Smith DA (1989) Molecular transformation of Fusarium solani with an antibiotic resistance marker having no fungal DNA homology. Curr Genet 15:421–428
Martín-Udíroz M, Madrid MP, Roncero MIG (2004) Role of chitin synthase genes in Fusarium oxysporum. Microbiology 150:3175–3187
Martín-Udíroz M, Roncero MIG, Gonzalez-Reyes JA, Ruiz-Roldán C (2008) ChsVb, a class VII chitin synthase involved in septation is critical for pathogenicity in Fusarium oxysporum. Eukaryot Cell 7:112–121
Mellado E, Dubreucq G, Mol P, Sarfati J, Paris S, Diaquin M, Holden DW, Rodriguez-Tudela JL, Latgé JP (2003) Cell wall biogenesis in a double chitin synthase mutant (chsG − /chsE −) of Aspergillus fumigatus. Fungal Genet Biol 38:98–109
Morgan WTJ, Elson LA (1934) A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem J 28:988–995
Munkvold GP, Desjardins AE (1997) Fumonisins in maize: can we reduce their occurrence? Plant Dis 81:556–565
Munro CA, Gow NAR (2001) Chitin synthesis in human pathogenic fungi. Med Mycol 39:41–53
Nagahashi S, Sudoh M, Ono N, Sawada R, Yamaguchi E, Uchida Y, Mio T, Takagi M, Arisawa M, Yamada-Okabe H (1995) Characterization of chitin synthase 2 of Saccharomyces cerevisiae. Implication of two highly conserved domains as possible catalytic sites. J Biol Chem 270:13961–13967
Niño-Vega GA, Carrero L, San-Blas G (2004) Isolation of the CHS4 gene of Paracoccidioides brasiliensis and its accommodation in a new class of chitin synthases. Med Mycol 42:51–57
Plattner RD (1995) Detection of fumonisins produced in Fusarium moniliforme cultures by HPLC with electrospray MS and evaporative light scattering detectors. Nat Toxins 3:294–298
Proctor RH, Desjardins AE, Plattner RD, Hohn TM (1999) A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi mating population A. Fungal Genet Biol 27:100–112
Richmond T (2000) Higher plant cellulose synthases. Genome Biol 1:3001–3005
Roncero C (2002) The genetic complexity of chitin synthesis in fungi. Curr Genet 41:367–378
Ruiz-Herrera J, Gonzalez-Prieto JM, Ruiz-Medrano R (2002) Evolution and phylogenetic relationships of chitin synthases from yeasts and fungi. FEMS Yeast Res 1:247–256
Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Seefelder W, Humpf HU, Schwerdt G, Freudinger R, Gekle M (2003) Induction of apoptosis in cultured human proximal tubule cells by fumonisins and fumonisin metabolites. Toxicol Appl Pharmacol 192:146–153
Shaw JA, Mol PC, Bowers B, Silverman SJ, Valdivieso MH, Duran A, Cabib E (1991) The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J Cell Biol 114:111–123
Silverman SJ, Sburlati A, Slater ML, Cabib E (1988) Chitin synthase 2 is essential for septum formation and cell division in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 85:4735–4739
Soulié MC, Piffeteau A, Choquer M, Boccara M, Vidal-Cros A (2003) Disruption of Botrytis cinerea class I chitin synthase gene Bcchs1 results in cell wall weakening and reduced virulence. Fungal Genet Biol 40:38–46
Soulié MC, Perino C, Piffeteau A, Choquer M, Malfatti P, Cimerman A, Kunz C, Boccara M, Vidal-Cross A (2006) Botrytis cinerea virulence is drastically reduced after disruption of chitin synthase class III gene (Bcchs3a). Cell Microbiol 8:1310–1321
Takeshita N, Ohta A, Horiuchi H (2002) csmA, a gene encoding a class V chitin synthase with a myosin-like domain of Aspergillus nidulans, is translated as a single polypeptide and regulated in response to osmotic conditions. Biochem Biophys Res Commun 298:103–109
Takeshita N, Yamashita S, Ohta A, Horuchi H (2006) Aspergillus nidulans class V and VI chitin synthases CsmA and CsmB, each with a myosin motor-like domain, perform compensatory functions that are essential for hyphal tip growth. Mol Microbiol 59:1380–1394
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis. (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Turgeon BG, Garber RC, Yoder OC (1987) Development of a fungal transformation system based on selection of sequences with promoter activity. Mol Cell Biol 7:3297–3305
Vidal-Cros A, Boccara M (1998) Identification of four chitin synthase genes in the rice blast disease agent Magnaporthe grisea. FEMS Microbiol Lett 165:103–109
Walker JE, Saraste M, Runswick MJ, Gay NJ (1982) Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases, and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J 1:945–951
Weber I, Aßmann D, Thines E, Steinberg G (2006) Polar localizing Class V myosin chitin synthases are essential during early plant infection in the plant pathogenic fungus Ustilago maydis. Plant Cell 18:225–242
Werner S, Sugui JA, Steinber G, eising HB (2007) A chitin synthase with a myosin-like motor domain is essential for hyphal growth, appressorium differentiation, and pathogenicity of the maize anthracnose fungus Colletotrichum graminicola. Mol Plant Microbe Interact 12:1555–1567
Wicklow DT, Horn BW, Shotwell OL, Hesseltine CW, Caldwell RW (1988) Fungal interference with Aspergillus flavus infection and aflatoxin contamination of maize grown in a controlled environment. Phytopathology 78:68–74
Yates IE, Bacon CW, Hinton DM (1997) Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis 81:723–728
Acknowledgments
We would like to acknowledge Amber M. Anderson, Nathan Deppe, and Debbie Shane for technical assistance. The mention of firm names or trade products does not imply that they are endorsed or recommended by the US Department of Agriculture over the firms or similar products not mentioned.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Cormack.
Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
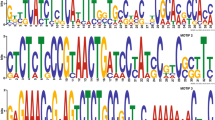
Alignment of conserved region 1 (Con1) found in the catalytic site of the eight putative F.verticillioides CHSs. Identical amino acids are shaded. Roman numerals above the alignment define the three sub domains (I–III) required for proper chitin synthase activity and classification. (TIFF 587 kb)
Rights and permissions
About this article
Cite this article
Larson, T.M., Kendra, D.F., Busman, M. et al. Fusarium verticillioides chitin synthases CHS5 and CHS7 are required for normal growth and pathogenicity. Curr Genet 57, 177–189 (2011). https://doi.org/10.1007/s00294-011-0334-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-011-0334-6