Abstract
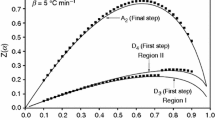
A macromonomer containing dual-lactone, MMDL, was characterized by thermal degradation kinetics and density functional theory (DFT) calculations. The frontier molecular orbitals, molecular structural geometry, molecular electrostatic potential (MEP) and electrostatic potential (ESP) maps were determined with the help of structure optimizations based on the DFT method with standard 3–21G* as a basis set that has polarization functions on the second row atoms only. The 3–21G* basis set comprises the same number of primitive Gaussian functions. The electronic properties, such as electron affinity, HOMO–LUMO energies, ionization energy, electronegativity, chemical potential, global hardness and softness, global electrophilicity were computed with the help of the DFT method. The MEP and ESP maps were determined to predict the reactive sites of the macromonomer. Finally, the activation energy and thermal degradation mechanism for the initial part of the decomposition process under non-isothermal conditions were determined from the thermogravimetric analysis by integral approximation methods. The decomposition activation energies of the macromonomer were computed with the help of Flynn–Wall–Ozawa, Coats–Redfern and Tang methods. The kinetic equations showed that the reaction mechanism was an R1 mechanism, which is a phase boundary-controlled reaction (one-dimensional movement) solid-state mechanism.








Similar content being viewed by others
References
Aggour YA, Abdel-Razik EA (1999) Graft copolymerization of end allenoxy polyoxyethylene macromonomer onto ethyl cellulose in a homogeneous system. Eur Polym J 35(12):2225–2228
Tsukahara Y (1996) In: Salamone JC (ed) Polymeric materials encyclopedia, vol 6. CRC Press, Boca Raton, p 3918
Gnanou Y (1996) In: Salamone JC (ed) Polymeric materials encyclopedia, vol 6. CRC Press, Boca Raton, p 3933
Mecerreyes D, Atthoff B, Boduch KA, Trollsås M, Hedrick JL (1999) Unimolecular combination of an atom transfer radical polymerization initiator and a lactone monomer as a route to new graft copolymers. Macromolecules 32(16):5175–5182
Dubois P, Degée P, Jérôme R, Teyssié P (1993) Macromolecular engineering of polylactones and polylactides. 11. Synthesis and use of alkylaluminum dialkoxides and dithiolates, as promoters of hydroxy telechelic poly(epsilon.-caprolactone) and .alpha., omega.-dihydroxy triblock copolymers containing outer polyester blocks. Macromolecules 26(11):2730–2735
Pantazis D, Chalari I, Hadjichristidis N (2003) Anionic polymerization of styrenic macromonomers. Macromolecules 36(11):3783–3785
Mecerreyes D, Pomposo JA, Bengoetxea M, Grande H (2000) Novel pyrrole end-functional macromonomers prepared by ring-opening and atom-transfer radical polymerizations. Macromolecules 33(16):5846–5849
Amass W, Amass A, Tighe B (1998) A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym Int 47(2):89–144
Kulkarni RK, Pani KC, Neuman C, Leonard F (1966) Polylactic acid for surgical implants. Arch Surg Chic 93(5):839–843
Gilding DK, Reed AM (1979) Biodegradable polymers for use in surgery—polyglycolic/poly(actic acid) homo-and copolymers: 1. Polymer 20(12):1459–1464
Middleton JC, Tipton AJ (1998) Synthetic biodegradable polymers as medical devices. Med Plast Biomater 5:30–39
Albertsson AC, Varma IK (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4(6):1466–1486
Kurt A (2009) Thermal decomposition kinetics of poly (nButMA-b-St) diblock copolymer synthesized by ATRP. J Appl Polym Sci 114(1):624–629
Bezgin F, Demirelli K (2016) Synthesis, characterization and thermal degradation kinetics of photoresponsive graft copolymers. J Thermoplast Compos Mater 29(8):1135–1150
Papageorgiou GZ, Tsanaktsis V, Papageorgiou DG, Chrissafis K, Exarhopoulos S, Bikiaris DN (2015) Furan-based polyesters from renewable resources: crystallization and thermal degradation behavior of poly(hexamethylene 2,5-furan-dicarboxylate). Eur Polym J 67:383–396
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520(1):1–19
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Demirelli K, Bezgin F (2012) Synthesis and characterization of lactone functional macromonomers by end group deactivation and their use in miktoarm star polymer. OJP Chem 2:42–55
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2010) Gaussian, Inc., Wallingford CT
Dennington R, Keith T, Millam J (2010) GaussView, version 5. Semichem Inc., Shawnee Mission
Gordon MS (1980) The isomers of silacyclopropane. Chem Phys Lett 76(1):163–168
Peterson JD, Vyazovkin S, Wight CA (2001) Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and poly (propylene). Macromol Chem Phys 202(6):775–784
Flynn JH, Wall LA (1966) General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand 70(6):487–523
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38(11):1881–1886
Doyle CD (1965) Series approximations to the equation of thermogravimetric data. Nature 207:290–291
Tang W, Liu Y, Zhang CH, Wang C (2003) New approximate formula for Arrhenius temperature integral. Thermochim Acta 408:39
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Asmadi A, Neumann MA, Kendrick J, Girard P, Perrin MA, Leusen FJ (2009) Revisiting the blind tests in crystal structure prediction: accurate energy ranking of molecular crystals. J Phys Chem B 113(51):16303–16313
Chan HC, Kendrick J, Leusen FJ (2011) Molecule VI, a benchmark crystal-structure-prediction sulfonimide: are its polymorphs predictable? Angew Chem Int Ed 50(13):2979–2981
Ma S, Hill JO, Heng S (1991) A kinetic analysis of the pyrolysis of some Australian coals by nonisothermal thermogravimetry. J Therm Anal 37(6):1161–1177
Jimenez A, Berenguer V, Lopez J, Sanchez A (1993) Thermal degradation study of poly(vinylchloride): kinetic analysis of thermogravimetric data. J Appl Polym Sci 50(9):1565–1573
Gunasekaran S, Balaji RA, Kumeresan S, Anand G, Srinivasan S (2008) Experimental and theoretical investigations of spectroscopic properties of N-acetyl-5-methoxytryptamine. Can J Anal Sci Spectrosc 53:149–160
Fleming I (1976) Frontier orbitals and organic chemical reactions. Wiley, New York
Padmaja L, Ravikumar C, Sajan D, Hubert Joe I, Jayakumar VS, Pettit GR, Faurskov Nielsen O (2009) Density functional study on the structural conformations and intramolecular charge transfer from the vibrational spectra of the anticancer drug combretastatin-A2. J Raman Spectrosc 40(4):419–428
Sagdinc S, Pir H (2009) Spectroscopic and DFT studies of flurbiprofen as dimer and its Cu(II) and Hg(II) complexes. Spectrochim Acta Part A Mol Biomol Spectrosc 73(1):181–194
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121(9):1922–1924
Chattaraj PK, Maiti B, Sarkar U (2003) Philicity: a unified treatment of chemical reactivity and selectivity. J Phys Chem A 107(25):4973–4975
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68(8):3801–3807
Scrocco E, Tomasi J (1973) The electrostatic molecular potential as a tool for the interpretation of molecular properties. In: Davison A, Dewar MJS (eds) New concepts II. Current Chemistry, vol 42. Springer, Berlin, pp 95–170
Acknowledgements
The authors wish to thank Bitlis Eren University for furnishing the funds to obtain the Gaussian software used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akman, F., Duran, A. Thermal degradation kinetics and density functional study of a macromonomer containing dual-lactone. Polym. Bull. 75, 2953–2969 (2018). https://doi.org/10.1007/s00289-017-2198-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2198-5