Abstract
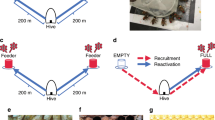
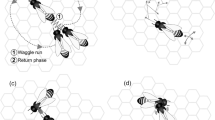
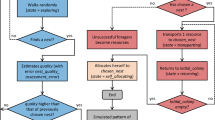
Honey bees make decisions regarding foraging and nest-site selection in groups ranging from hundreds to thousands of individuals. To effectively make these decisions, bees need to communicate within a spatially distributed group. However, the spatiotemporal dynamics of honey bee communication have been mostly overlooked in models of collective decisions, focusing primarily on mean field models of opinion dynamics. We analyze how the spatial properties of the nest or hive, and the movement of individuals with different belief states (uncommitted or committed) therein affect the rate of information transmission using spatially-extended models of collective decision-making within a hive. Honeybees waggle-dance to recruit conspecifics with an intensity that is a threshold nonlinear function of the waggler concentration. Our models range from treating the hive as a chain of discrete patches to a continuous line (long narrow hive). The combination of population-thresholded recruitment and compartmentalized populations generates tradeoffs between rapid information propagation with strong population dispersal and recruitment failures resulting from excessive population diffusion and also creates an effective colony-level signal-detection mechanism whereby recruitment to low quality objectives is blocked.










Similar content being viewed by others
Data availibility
Code for producing figures is available at https://github.com/sbidari/hivegeom
Notes
Further down, this can be seen in Fig. 4f, g, which demonstrates that recruitment in peripheral patches begins sooner for a significantly lower than critical value of \(D_v\).
References
Bidari S, Peleg O, Kilpatrick ZP (2019) Social inhibition maintains adaptivity and consensus of honeybees foraging in dynamic environments. R Soc Open Sci 6(12):191681
Biesmeijer JC, de Vries H (2001) Exploration and exploitation of food sources by social insect colonies: a revision of the scout-recruit concept. Behav Ecol Sociobiol 49(2–3):89–99
Biesmeijer JC, Seeley TD (2005) The use of waggle dance information by honey bees throughout their foraging careers. Behav Ecol Sociobiol 59(1):133–142
Bonabeau E, Theraulaz G, Deneubourg J-L (1996) Quantitative study of the fixed threshold model for the regulation of division of labour in insect societies. Proc R Soc Lond Ser B Biol Sci 263(1376):1565–1569
Burd M, Shiwakoti N, Sarvi M, Rose G (2010) Nest architecture and traffic flow: large potential effects from small structural features. Ecol Entomol 35(4):464–468
Camazine S, Deneubourg J-L, Franks NR, Sneyd J, Bonabeau E, Theraula G (2003) Self-organization in biological systems. Princeton University Press, Princeton
Clark CW, Mangel M (1986) The evolutionary advantages of group foraging. Theor Popul Biol 30(1):45–75
Davidson JD, Arauco-Aliaga RP, Crow S, Gordon DM, Goldman MS (2016) Effect of interactions between harvester ants on forager decisions. Front Ecol Evol 4:115
Detrain C, Deneubourg J-L (2006) Self-organized structures in a superorganism: do ants “behave” like molecules? Phys Life Rev 3(3):162–187
Dornhaus A, Klügl F, Oechslein C, Puppe F, Chittka L (2006) Benefits of recruitment in honey bees: effects of ecology and colony size in an individual-based model. Behav Ecol 17(3):336–344. https://doi.org/10.1093/beheco/arj036
Dreller C (1998) Division of labor between scouts and recruits: genetic influence and mechanisms. Behav Ecol Sociobiol 43(3):191–196
Edge AA, van Nest BN, Johnson JN, Miller SN, Naeger N, Boyd SD, Moore D (2012) Diel nectar secretion rhythm in squash (cucurbita pepo) and its relation with pollinator activity. Apidologie 43(1):1–16
Franks NR, Pratt SC, Mallon EB, Britton NF, Sumpter DJ (2002) Information flow, opinion polling and collective intelligence in house-hunting social insects. Philos Trans R Soc Lond Ser B Biol Sci 357(1427):1567–1583
Grüter C, Farina WM (2009) The honeybee waggle dance: can we follow the steps? Trends Ecol Evol 24(5):242–247
Janson CH (1990) Ecological consequences of individual spatial choice in foraging groups of brown capuchin monkeys, cebus apella. Anim Behav 40(5):922–934
Johnson BR (2003) Organization of work in the honeybee: a compromise between division of labour and behavioural flexibility. Proc R Soc Lond Ser B Biol Sci 270(1511):147–152
Karsai M, Kivelä M, Pan RK, Kaski K, Kertész J, Barabási A-L, Saramäki J (2011) Small but slow world: How network topology and burstiness slow down spreading. Phys Rev E 83(2):025102
Krause J, Ruxton GD, Ruxton G, Ruxton IG et al (2002) Living in groups. Oxford University Press, Oxford
Leavell BC, Bernal XE (2019) The cognitive ecology of stimulus ambiguity: a predator-prey perspective. Trends Ecol Evol 34(11):1048–1060
Leonard AS, Dornhaus A, Papaj DR (2011) Flowers help bees cope with uncertainty: signal detection and the function of floral complexity. J Exp Biol 214(1):113–121
Mailleux A-C, Detrain C, Deneubourg J-L (2006) Starvation drives a threshold triggering communication. J Exp Biol 209(21):4224–4229
Mateo D, Horsevad N, Hassani V, Chamanbaz M, Bouffanais R (2019) Optimal network topology for responsive collective behavior. Science advances 5(4):eaau0999
Pagliara R, Gordon DM, Leonard NE (2018) Regulation of harvester ant foraging as a closed-loop excitable system. PLoS Comput Biol 14(12):e1006200e1006200
Passino KM, Seeley TD, Visscher PK (2008) Swarm cognition in honey bees. Behav Ecol Sociobiol 62(3):401–414
Pinter-Wollman N (2015) Nest architecture shapes the collective behaviour of harvester ants. Biol Let 11(10):20150695
Pless E, Queirolo J, Pinter-Wollman N, Crow S, Allen K, Mathur MB, Gordon DM (2015) Interactions increase forager availability and activity in harvester ants. PLoS ONE 10(11):e141971
Price R, Dulex N, Vial N, Vincent C, Grüter C (2019) Honeybees forage more successfully without the “dance language” in challenging environments. Science advances 5(2):eaat0450
Raihan J, Kawakubo N (2014) Nondestructive and continuous observation of nectar volume using time-interval photography. Plant Species Biol 29(2):212–215
Razin N, Eckmann J-P, Feinerman O (2013) Desert ants achieve reliable recruitment across noisy interactions. J R Soc Interface 10(82):20130079
Reina A, Marshall JA, Trianni V, Bose T (2017) Model of the best-of-n nest-site selection process in honeybees. Phys Rev E 95(5):052411
Ribbands CR (1952) Division of labour in the honeybee community. Proc R Soc Lond Ser B Biol Sci 140(898):32–43
Sasaki T, Pratt SC (2018) The psychology of superorganisms: collective decision making by insect societies. Annu Rev Entomol 63:259–275
Seeley TD (1983) Division of labor between scouts and recruits in honeybee foraging. Behav Ecol Sociobiol 12(3):253–259
Seeley TD (1986) Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behav Ecol Sociobiol 19(5):343–354
Seeley TD (1995) The wisdom of the hive. Harvard University Press, Cambridge
Seeley TD (2010) Honeybee democracy. Princeton University Press, Princeton
Seeley TD, Towne WF (1992) Tactics of dance choice in honey bees: do foragers compare dances? Behav Ecol Sociobiol 30(1):59–69
Seeley TD, Visscher PK (1988) Assessing the benefits of cooperation in honeybee foraging: search costs, forage quality, and competitive ability. Behav Ecol Sociobiol 22(4):229–237
Seeley TD, Visscher PK, Schlegel T, Hogan PM, Franks NR, Marshall JA (2012) Stop signals provide cross inhibition in collective decision-making by honeybee swarms. Science 335(6064):108–111
Sumpter D, Pratt S (2003) A modelling framework for understanding social insect foraging. Behav Ecol Sociobiol 53(3):131–144
Tautz J (1996) Honeybee waggle dance: recruitment success depends on the dance floor. J Exp Biol 199(6):1375–1381
Tautz J, Lindauer M (1997) Honeybees establish specific sites on the comb for their waggle dances. J Comp Physiol A 180(5):537–539
Verghese P (2001) Visual search and attention: a signal detection theory approach. Neuron 31(4):523–535
Von Frisch K (1967) The dance language and orientation of bees
Wilkinson GS, Boughman JW (1998) Social calls coordinate foraging in greater spear-nosed bats. Anim Behav 55(2):337–350
Acknowledgements
SB and ZPK were supported by an NIH (R01MH115557) and NSF (DMS-1853630) grants. SB was also supported by a Dissertation Fellowship from the American Association of University Women.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bidari, S., Kilpatrick, Z.P. Hive geometry shapes the recruitment rate of honeybee colonies. J. Math. Biol. 83, 20 (2021). https://doi.org/10.1007/s00285-021-01644-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-021-01644-9