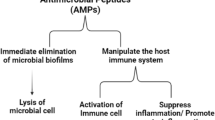
Abstract
Antimicrobial peptides (AMPs) are small-molecule peptides that play a vital role in the nonspecific immune defense system of organisms. They mainly kill microorganisms by physically destroying the cell membrane and causing the leakage of contents. AMPs have attracted much attention as potential alternatives to antibiotics due to their low susceptibility to resistance. Streptococcus mutans (S. mutans) is one of the main causative agents of human dental caries. The design, screening, and efficacy evaluation of AMPs targeting S. mutans offer new possibilities for the prevention and treatment of oral diseases, especially dental caries, in the future. This article reviews AMPs from different sources that have inhibitory effects on S. mutans, discusses the mechanism of action of AMPs against S. mutans biofilms, and focuses on the research progress of screening methods, design modification, and biological activity evaluation of AMPs. We hope to provide insights and reference value for the development of new biologics.


Similar content being viewed by others
Abbreviations
- AI :
-
Artificial intelligence
- AMPs :
-
Antimicrobial peptides
- ATP :
-
Adenosine triphosphate
- CAT :
-
Catalytic domain
- CSP :
-
Competence-stimulating peptide ComC
- EPS :
-
Extracellular polysaccharides
- EtBr :
-
Ethidium bromide
- GA :
-
Gallic acid
- GBD :
-
Glucan-binding domain
- Gbps :
-
Glucan-binding proteins
- Gram + :
-
Gram-positive bacteria
- Gram − :
-
Gram-negative bacteria
- GTFs :
-
Glucosyltransferases
- hBD :
-
Human beta-defensins
- HK :
-
Histidine kinase
- HMP :
-
Human Microbiome Project
- HNPs :
-
Human neutrophil peptides
- IC50 :
-
Half maximal inhibitory concentration
- LPS :
-
Lipopolysaccharide
- MBC :
-
Minimum bactericidal concentration
- MIC :
-
Minimum inhibitory concentration
- PAc :
-
Protein antigen c
- PI :
-
Polyphemusin I
- P regions :
-
Proline-rich repeat regions
- QS :
-
Quorum sensing
- rDNA :
-
Ribosomal DNA
- S. mutans :
-
Streptococcus mutans
- Sp :
-
Phosphorylated serine
- TCSTS :
-
Two-component signal transduction system
- TF-κB :
-
Nuclear transcription factor-κB
- TiBP :
-
Titanium-binding peptide
- TNF-α :
-
Tumor necrosis factor alpha
- RR :
-
Response regulator
References
Lazzaro BP, Zasloff M, Rolff J (2020) Antimicrobial peptides: application informed by evolution. Science. https://doi.org/10.1126/science.aau5480
Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ (2020) Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov 19:311–332. https://doi.org/10.1038/s41573-019-0058-8
da Silva BR, Conrado AJS, Pereira AL, Evaristo FFV, Arruda FVS, Vasconcelos MA, Lorenzón EN, Cilli EM, Teixeira EH (2017) Antibacterial activity of a novel antimicrobial peptide [W7]KR12-KAEK derived from KR-12 against Streptococcus mutans planktonic cells and biofilms. Biofouling 33:835–846. https://doi.org/10.1080/08927014.2017.1374378
Jiang W, Wang Y, Luo J, Chen X, Zeng Y, Li X, Feng Z, Zhang L (2020) Antimicrobial peptide GH12 prevents dental caries by regulating dental plaque microbiota. Appl Environ Microbiol. https://doi.org/10.1128/aem.00527-20
Capasso C, Supuran CT (2016) An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr Top Med Chem 16:2359–2368. https://doi.org/10.2174/1568026616666160413135522
Ribeiro TR, Dria KJ, de Carvalho CBM, Monteiro AJ, Fonteles MC, de Moraes CK, Fonteles CSR (2013) Salivary peptide profile and its association with early childhood caries. Int J Pediatr Dent 23:225–234. https://doi.org/10.1111/j.1365-263X.2012.01258.x
Liu Y, Kamesh AC, Xiao Y, Sun V, Hayes M, Daniell H, Koo H (2016) Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials 105:156–166. https://doi.org/10.1016/j.biomaterials.2016.07.042
Jug M, Kosalec I, Maestrelli F, Mura P (2012) Development of low methoxy amidated pectin-based mucoadhesive patches for buccal delivery of triclosan: effect of cyclodextrin complexation. Carbohyd Polym 90:1794–1803. https://doi.org/10.1016/j.carbpol.2012.07.074
Su M, Yao S, Gu L, Huang Z, Mai S (2018) Antibacterial effect and bond strength of a modified dental adhesive containing the peptide nisin. Peptides 99:189–194. https://doi.org/10.1016/j.peptides.2017.10.003
Consortium HMP (2012) Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. https://doi.org/10.1038/nature11234
Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR 3rd, Heydorn A, Koo H (2012) The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog 8:e1002623. https://doi.org/10.1371/journal.ppat.1002623
Singh N, Das PP (2022) Microbial biofilms. Core Microbiome 54:249–267
Matsumoto-Nakano M (2018) Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dental Sci Rev 54:22–29. https://doi.org/10.1016/j.jdsr.2017.08.002
Álvarez S, Leiva-Sabadini C, Schuh C, Aguayo S (2022) Bacterial adhesion to collagens: implications for biofilm formation and disease progression in the oral cavity. Crit Rev Microbiol 48:83–95. https://doi.org/10.1080/1040841x.2021.1944054
Bowen WH, Koo H (2011) Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res 45:69–86. https://doi.org/10.1159/000324598
Lynch DJ, Fountain TL, Mazurkiewicz JE, Banas JA (2007) Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol Lett 268:158–165. https://doi.org/10.1111/j.1574-6968.2006.00576.x
Yadav P, Verma S, Bauer R, Kumari M, Dua M, Johri AK, Yadav V, Spellerberg B (2020) Deciphering streptococcal biofilms. Microorganisms. https://doi.org/10.3390/microorganisms8111835
Matsumoto-Nakano M, Tsuji M, Amano A, Ooshima T (2008) Molecular interactions of alanine-rich and proline-rich regions of cell surface protein antigen c in Streptococcus mutans. Oral Microbiol Immunol 23:265–270. https://doi.org/10.1111/j.1399-302X.2007.00421.x
Aviles-Reyes A, Miller JH, Simpson-Haidaris PJ, Lemos JA, Abranches J (2014) Cnm is a major virulence factor of invasive Streptococcus mutans and part of a conserved three-gene locus. Mol Oral Microbiol 29:11–23. https://doi.org/10.1111/mom.12041
Kaur G, Balamurugan P, Princy SA (2017) Inhibition of the quorum sensing system (ComDE pathway) by aromatic 1,3-di-m-tolylurea (DMTU): cariostatic effect with fluoride in wistar rats. Front Cell Infect Microbiol 7:313. https://doi.org/10.3389/fcimb.2017.00313
Di Salle A, Viscusi G, Di Cristo F, Valentino A, Gorrasi G, Lamberti E, Vittoria V, Calarco A, Peluso G (2021) Antimicrobial and antibiofilm activity of curcumin-loaded electrospun nanofibers for the prevention of the biofilm-associated infections. Molecules. https://doi.org/10.3390/molecules26164866
Liu S, Sun Y, Liu Y, Hu F, Xu L, Zheng Q, Wang Q, Zeng G, Zhang K (2022) Genomic and phenotypic characterization of Streptococcus mutans isolates suggests key gene clusters in regulating its interaction with Streptococcus gordonii. Front Microbiol 13:945108. https://doi.org/10.3389/fmicb.2022.945108
Schaefers MM (2020) Regulation of virulence by two-component systems in pathogenic Burkholderia. Infect Immun. https://doi.org/10.1128/iai.00927-19
Li YuW, Ming Chen J, Sun Y, Geng XP (2017) Targeting two-component signal-transduction systems of bacteria for go od potential drug design. J Intensive Crit Care 2:210. https://doi.org/10.21767/2471-8505.100012
Wang W-L, Liu J, Huo Y-B, Ling J-Q (2013) Bacteriocin immunity proteins play a role in quorum-sensing system regulated antimicrobial sensitivity of Streptococcus mutans UA159. Arch Oral Biol 58:384–390. https://doi.org/10.1016/j.archoralbio.2012.09.001
Matsumoto-Nakano M, Kuramitsu HK (2006) Role of bacteriocin immunity proteins in the antimicrobial sensitivity of Streptococcus mutans. J Bacteriol 188:8095–8102. https://doi.org/10.1128/JB.00908-06
Wei Y, Sturges CI, Palmer KL (2023) Human serum supplementation promotes streptococcus mitis growth and induces specific transcriptomic responses. Microbiol Spectr 11:e0512922. https://doi.org/10.1128/spectrum.05129-22
He L-Y, Le Y-J, Guo Z, Li S, Yang X-Y (2021) The role and regulatory network of the CiaRH two-component system in streptococcal species. Front Microbiol. https://doi.org/10.3389/fmicb.2021.693858
Lévesque CM, Mair RW, Perry JA, Lau PC, Li YH, Cvitkovitch DG (2007) Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett Appl Microbiol 45:398–404. https://doi.org/10.1111/j.1472-765X.2007.02203.x
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395. https://doi.org/10.1038/415389a
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250. https://doi.org/10.1038/nrmicro1098
Niu JY, Yin IX, Wu WKK, Li Q-L, Mei ML, Chu CH (2021) Antimicrobial peptides for the prevention and treatment of dental caries: a concise review. Arch Oral Biol 122:105022. https://doi.org/10.1016/j.archoralbio.2020.105022
Luong AD, Buzid A, Luong JHT (2022) Important roles and potential uses of natural and synthetic antimicrobial peptides (AMPs) in oral diseases: cavity, periodontal disease, and thrush. J Funct Biomater 13:175
Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720. https://doi.org/10.1038/nri1180
Zhai YJ, Feng Y, Ma X, Ma F (2023) Defensins: defenders of human reproductive health. Hum Reprod Update 29:126–154. https://doi.org/10.1093/humupd/dmac032
Wuersching SN, Huth KC, Hickel R, Kollmuss M (2021) Inhibitory effect of LL-37 and human lactoferricin on growth and biofilm formation of anaerobes associated with oral diseases. Anaerobe 67:102301. https://doi.org/10.1016/j.anaerobe.2020.102301
Zeth K, Sancho-Vaello E (2021) Structural plasticity of LL-37 indicates elaborate functional adaptation mechanisms to bacterial target structures. Int J Mol Sci. https://doi.org/10.3390/ijms22105200
Wuersching SN, Huth KC, Hickel R, Kollmuss M (2021) Targeting antibiotic tolerance in anaerobic biofilms associated with oral diseases: Human antimicrobial peptides LL-37 and lactoferricin enhance the antibiotic efficacy of amoxicillin, clindamycin and metronidazole. Anaerobe 71:102439. https://doi.org/10.1016/j.anaerobe.2021.102439
Zolin GVS, Fonseca FHD, Zambom CR, Garrido SS (2021) Histatin 5 metallopeptides and their potential against Candida albicans pathogenicity and drug resistance. Biomolecules. https://doi.org/10.3390/biom11081209
Mason AJ, Bertani P, Moulay G, Marquette A, Perrone B, Drake AF, Kichler A, Bechinger B (2007) Membrane interaction of chrysophsin-1, a histidine-rich antimicrobial peptide from red sea bream. Biochemistry 46:15175–15187. https://doi.org/10.1021/bi701344m
Aa-S K, Hozzein WN (2022) Antibacterial activities of two potential peptides extracted from Polistes wattii Cameron, 1900 (Vespidae: Polistinae) wasp venom collected at Eastern Province. Saudi Arabia PLoS One 17:e0264035. https://doi.org/10.1371/journal.pone.0264035
Cai S, Meng K, Liu P, Cao X, Wang G (2021) Suppressive effects of gecko cathelicidin on biofilm formation and cariogenic virulence factors of Streptococcus mutans. Arch Oral Biol 129:105205. https://doi.org/10.1016/j.archoralbio.2021.105205
Kokilakanit P, Koontongkaew S, Roytrakul S, Utispan K (2020) A novel non-cytotoxic synthetic peptide, Pug-1, exhibited an antibiofilm effect on Streptococcus mutans adhesion. Lett Appl Microbiol 70:151–158. https://doi.org/10.1111/lam.13265
Bédard F, Biron E (2018) Recent progress in the chemical synthesis of class II and S-glycosylated bacteriocins. Front Microbiol 9:1048. https://doi.org/10.3389/fmicb.2018.01048
Lopes SR, Matuda AGN, Campos RP, Mafetano A, Barnabe AHM, Chagas GS, Barcellos DC, Niu LN, Tay FR, Pucci CR (2022) Development of an antibacterial dentin adhesive. Polymers. https://doi.org/10.3390/polym14122502
Kawada-Matsuo M, Komatsuzawa H (2017) Role of Streptococcus mutans two-component systems in antimicrobial peptide resistance in the oral cavity. Jpn Dental Sci Rev 53:86–94. https://doi.org/10.1016/j.jdsr.2016.12.002
Yamakami K, Tsumori H, Sakurai Y, Shimizu Y, Nagatoshi K, Sonomoto K (2013) Sustainable inhibition efficacy of liposome-encapsulated nisin on insoluble glucan-biofilm synthesis by Streptococcus mutans. Pharm Biol 51:267–270. https://doi.org/10.3109/13880209.2012.717227
Tanhaieian A, Pourgonabadi S, Akbari M, Mohammadipour HS (2020) The effective and safe method for preventing and treating bacteria-induced dental diseases by herbal plants and a recombinant peptide. J Clin Exp Dent 12:e523–e532. https://doi.org/10.4317/jced.55717
Tossi A, Sandri L, Giangaspero A (2000) Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4–30. https://doi.org/10.1002/1097-0282(2000)55:1%3c4::Aid-bip30%3e3.0.Co;2-m
Wiradharma N, Khoe U, Hauser CAE, Seow SV, Zhang S, Yang Y-Y (2011) Synthetic cationic amphiphilic α-helical peptides as antimicrobial agents. Biomaterials 32:2204–2212. https://doi.org/10.1016/j.biomaterials.2010.11.054
Gan BH, Gaynord J, Rowe SM, Deingruber T, Spring DR (2021) The multifaceted nature of antimicrobial peptides: current synthetic chemistry approaches and future directions. Chem Soc Rev 50:7820–7880. https://doi.org/10.1039/d0cs00729c
Zhou XR, Zhang Q, Tian XB, Cao YM, Liu ZQ, Fan R, Ding XF, Zhu Z, Chen L, Luo SZ (2016) From a pro-apoptotic peptide to a lytic peptide: one single residue mutation. Biochim Biophys Acta 1858:1914–1925. https://doi.org/10.1016/j.bbamem.2016.05.012
Chen L, Jia L, Zhang Q, Zhou X, Liu Z, Li B, Zhu Z, Wang F, Yu C, Zhang Q, Chen F, Luo S-Z (2017) A novel antimicrobial peptide against dental-caries-associated bacteria. Anaerobe 47:165–172. https://doi.org/10.1016/j.anaerobe.2017.05.016
Ito T, Ichinosawa T, Shimizu T (2017) Streptococcal adhesin SspA/B analogue peptide inhibits adherence and impacts biofilm formation of Streptococcus mutans. PLoS ONE 12:e0175483. https://doi.org/10.1371/journal.pone.0175483
Chen Z, Yang G, Lu S, Chen D, Fan S, Xu J, Wu B, He J (2019) Design and antimicrobial activities of LL-37 derivatives inhibiting the formation of Streptococcus mutans biofilm. Chem Biol Drug Des 93:1175–1185. https://doi.org/10.1111/cbdd.13419
Zhang P, Wu S, Li J, Bu X, Dong X, Chen N, Li F, Zhu J, Sang L, Zeng Y, Liang S, Yu Z, Liu Z (2022) Dual-sensitive antibacterial peptide nanoparticles prevent dental caries. Theranostics 12:4818–4833. https://doi.org/10.7150/thno.73181
Liang D, Li H, Xu X, Liang J, Dai X, Zhao W (2019) Design, screening and antimicrobial activity of novel peptides against Streptococcus mutans. Nan Fang Yi Ke Da Xue Xue Bao 39:823–829. https://doi.org/10.12122/j.issn.1673-4254.2019.07.12
Liang J, Liang D, Liang Y, He J, Zuo S, Zhao W (2021) Effects of a derivative of reutericin 6 and gassericin A on the biofilm of Streptococcus mutans in vitro and caries prevention in vivo. Odontology 109:53–66. https://doi.org/10.1007/s10266-020-00529-5
Gautier R, Douguet D, Antonny B, Drin G (2008) HELIQUEST: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24:2101–2102. https://doi.org/10.1093/bioinformatics/btn392
He J, Liang D, Liang Y, Zuo S, Zhao W (2021) Design, screening and antibacterial activity evaluation of the novel antibacterial peptide KR-1. Nan Fang Yi Ke Da Xue Xue Bao 41:923–930. https://doi.org/10.12122/j.issn.1673-4254.2021.06.16
da Silva BR, de Freitas VAA, Carneiro VA, Arruda FVS, Lorenzón EN, de Aguiar ASW, Cilli EM, Cavada BS, Teixeira EH (2013) Antimicrobial activity of the synthetic peptide Lys-a1 against oral streptococci. Peptides 42:78–83. https://doi.org/10.1016/j.peptides.2012.12.001
Shang D, Liang H, Wei S, Yan X, Yang Q, Sun Y (2014) Effects of antimicrobial peptide L-K6, a temporin-1CEb analog on oral pathogen growth, Streptococcus mutans biofilm formation, and anti-inflammatory activity. Appl Microbiol Biotechnol 98:8685–8695. https://doi.org/10.1007/s00253-014-5927-9
Zhou L, Wong HM, Zhang YY, Li QL (2020) Constructing an Antibiofouling and Mineralizing Bioactive Tooth Surface to Protect against Decay and Promote Self-Healing. ACS Appl Mater Interfaces 12:3021–3031. https://doi.org/10.1021/acsami.9b19745
Zhou L, Li QL, Wong HM (2021) A novel strategy for caries management: constructing an antibiofouling and mineralizing dual-bioactive tooth surface. ACS Appl Mater Interfaces 13:31140–31152. https://doi.org/10.1021/acsami.1c06989
Zhang L-y, Fang Z-h, Q-l Li, Cao CY (2019) A tooth-binding antimicrobial peptide to prevent the formation of dental biofilm. J Mater Sci 30:45. https://doi.org/10.1007/s10856-019-6246-6
Niu JY, Yin IX, Wu WKK, Li Q-L, Mei ML, Chu CH (2022) Efficacy of the dual-action GA-KR12 peptide for remineralising initial enamel caries: an in vitro study. Clin Oral Invest 26:2441–2451. https://doi.org/10.1007/s00784-021-04210-1
Min KR, Galvis A, Williams B, Rayala R, Cudic P, Ajdic D (2017) Antibacterial and antibiofilm activities of a novel synthetic cyclic lipopeptide against cariogenic Streptococcus mutans UA159. Antimicrob Agents Chemother. https://doi.org/10.1128/aac.00776-17
Xiang S-W, Shao J, He J, Wu X-Y, Xu X-H, Zhao W-H (2019) A membrane-targeted peptide inhibiting PtxA of phosphotransferase system blocks Streptococcus mutans. Caries Res 53:176–193
Huo L, Huang X, Ling J, Liu H, Liu J (2018) Selective activities of STAMPs against Streptococcus mutans. Exp Ther Med 15:1886–1893. https://doi.org/10.3892/etm.2017.5631
Tian XLCC, Cyr K, Dong G, Salim H et al (2017) Targeted killing of Streptococcus mutans in biofilms by a pheromone guided antimicrobial peptide HP30. J Antibio Res 1(2):2018
Almaaytah A, Tarazi S, Al-Fandi M, Abuilhaija A, Al-shar’i N, Al-Balas Q, Abu-Awad A (2015) the design and functional characterization of the antimicrobial and antibiofilm activities of BMAP27-melittin, a rationally designed hybrid peptide. Int J Pept Res Ther 21:165–177. https://doi.org/10.1007/s10989-014-9444-6
Wang X, Wang Y, Wang K, Ren Q, Li H, Zheng S, Niu Y, Zhou X, Li W, Zhang L (2019) Bifunctional anticaries peptides with antibacterial and remineralizing effects. Oral Dis 25:488–496. https://doi.org/10.1111/odi.12990
Mai J, Tian X-L, Gallant Jeffrey W, Merkley N, Biswas Z, Syvitski R, Douglas Susan E, Ling J, Li Y-H (2011) A novel target-specific, salt-resistant antimicrobial peptide against the cariogenic pathogen Streptococcus mutans. Antimicrob Agents Chemother 55:5205–5213. https://doi.org/10.1128/AAC.05175-11
He J, Yarbrough DK, Kreth J, Anderson MH, Shi W, Eckert R (2010) Systematic approach to optimizing specifically targeted antimicrobial peptides against Streptococcus mutans. Antimicrob Agents Chemother 54:2143–2151. https://doi.org/10.1128/aac.01391-09
Baker JL, He X, Shi W (2019) Precision reengineering of the oral microbiome for caries management. Adv Dent Res 30:34–39. https://doi.org/10.1177/0022034519877386
Kaplan CW, Sim JH, Shah KR, Kolesnikova-Kaplan A, Shi W, Eckert R (2011) Selective membrane disruption: mode of action of C16G2, a specifically targeted antimicrobial peptide. Antimicrob Agents Chemother 55:3446–3452. https://doi.org/10.1128/aac.00342-11
Eckert R, Sullivan R, Shi W (2012) Targeted antimicrobial treatment to Re-establish a healthy microbial flora for long-term protection. Adv Dent Res 24:94–97. https://doi.org/10.1177/0022034512453725
Guo L, McLean JS, Yang Y, Eckert R, Kaplan CW, Kyme P, Sheikh O, Varnum B, Lux R, Shi W, He X (2015) Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci USA 112:7569–7574. https://doi.org/10.1073/pnas.1506207112
Guo L, Edlund A (2017) Targeted antimicrobial peptides: a novel technology to eradicate harmful Streptococcus Mutans. J Calif Dent Assoc 45:557–564
Tu H, Fan Y, Lv X, Han S, Zhou X, Zhang L (2016) Activity of synthetic antimicrobial peptide GH12 against oral streptococci. Caries Res 50:48–61. https://doi.org/10.1159/000442898
Wang Y, Fan Y, Zhou Z, Tu H, Ren Q, Wang X, Ding L, Zhou X, Zhang L (2017) De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch Oral Biol 80:41–50. https://doi.org/10.1016/j.archoralbio.2017.03.017
Zhang J, Chen C, Chen J, Zhou S, Zhao Y, Xu M, Xu H (2020) Dual mode of anti-biofilm action of G3 against Streptococcus mutans. ACS Appl Mater Interfaces 12:27866–27875. https://doi.org/10.1021/acsami.0c00771
Nguyen LT, Haney EF, Vogel HJ (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 29:464–472. https://doi.org/10.1016/j.tibtech.2011.05.001
Silva BRd, Freitas VAAd, Nascimento-Neto LG, Carneiro VA, Arruda FVS, Aguiar ASWd, Cavada BS, Teixeira EH (2012) Antimicrobial peptide control of pathogenic microorganisms of the oral cavity: a review of the literature. Peptides 36:315–321. https://doi.org/10.1016/j.peptides.2012.05.015
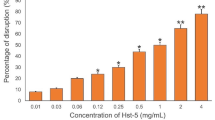
Huo L, Zhang K, Ling J, Peng Z, Huang X, Liu H, Gu L (2011) Antimicrobial and DNA-binding activities of the peptide fragments of human lactoferrin and histatin 5 against Streptococcus mutans. Arch Oral Biol 56:869–876. https://doi.org/10.1016/j.archoralbio.2011.02.004
Meir O, Zaknoon F, Cogan U, Mor A (2017) A broad-spectrum bactericidal lipopeptide with anti-biofilm properties. Sci Rep 7:2198. https://doi.org/10.1038/s41598-017-02373-0
Batoni G, Maisetta G, Esin S (2016) Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim Biophys Acta 1858:1044–1060. https://doi.org/10.1016/j.bbamem.2015.10.013
Robertson J, McGoverin C, Vanholsbeeck F, Swift S (2019) Optimisation of the protocol for the LIVE/DEAD(®) BacLight(TM) bacterial viability kit for rapid determination of bacterial load. Front Microbiol 10:801. https://doi.org/10.3389/fmicb.2019.00801
Jiang X, Wang Y, Li X, Feng Z, Zeng Y, Han S, Takahashi N, Zhang L (2022) Development and evaluation of a chewing gum containing antimicrobial peptide GH12 for caries prevention. Eur J Oral Sci 130:e12887. https://doi.org/10.1111/eos.12887
Xiong K, Chen X, Zhu H, Ji M, Zou L (2022) Anticaries activity of GERM CLEAN in Streptococcus mutans and Candida albicans dual-species biofilm. Oral Dis 28:829–839. https://doi.org/10.1111/odi.13799
Wisdom C, VanOosten SK, Boone KW, Khvostenko D, Arnold PM, Snead ML, Tamerler C (2016) Controlling the biomimetic implant interface: modulating antimicrobial activity by spacer design. J Mol Eng Mater. https://doi.org/10.1142/s2251237316400050
Röckendorf N, Nehls C, Gutsmann T (2022) Design of membrane active peptides considering multi-objective optimization for biomedical application. Membranes 12:180
Ma Y, Guo Z, Xia B, Zhang Y, Liu X, Yu Y, Tang N, Tong X, Wang M, Ye X, Feng J, Chen Y, Wang J (2022) Identification of antimicrobial peptides from the human gut microbiome using deep learning. Nat Biotechnol 40:921–931. https://doi.org/10.1038/s41587-022-01226-0
Huang DB, Brothers KM, Mandell JB, Taguchi M, Alexander PG, Parker DM, Shinabarger D, Pillar C, Morrissey I, Hawser S, Ghahramani P, Dobbins D, Pachuda N, Montelaro R, Steckbeck JD, Urish KL (2022) Engineered peptide PLG0206 overcomes limitations of a challenging antimicrobial drug class. PLoS ONE 17:e0274815. https://doi.org/10.1371/journal.pone.0274815
Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I (2001) Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol 31:1066–1075. https://doi.org/10.1002/1521-4141(200104)31:4%3c1066::aid-immu1066%3e3.0.co;2-#
Yang D, Biragyn A, Kwak LW, Oppenheim JJ (2002) Mammalian defensins in immunity: more than just microbicidal. Trends Immunol 23:291–296. https://doi.org/10.1016/s1471-4906(02)02246-9
Lee J-K, Chang SW, Perinpanayagam H, Lim S-M, Park Y-J, Han SH, Baek S-H, Zhu Q, Bae K-S, Kum K-Y (2013) Antibacterial efficacy of a human β-defensin-3 peptide on multispecies biofilms. Journal of Endodontics 39:1625–1629. https://doi.org/10.1016/j.joen.2013.07.035
Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC (1995) Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun 63:1291–1297. https://doi.org/10.1128/iai.63.4.1291-1297.1995
Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Tanaka S, Heumann D (2002) Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol 9:972–982. https://doi.org/10.1128/cdli.9.5.972-982.2002
Pirtskhalava M, Amstrong AA, Grigolava M, Chubinidze M, Alimbarashvili E, Vishnepolsky B, Gabrielian A, Rosenthal A, Hurt DE, Tartakovsky M (2021) DBAASP v3: database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res 49:D288–D297. https://doi.org/10.1093/nar/gkaa991
Shi G, Kang X, Dong F, Liu Y, Zhu N, Hu Y, Xu H, Lao X, Zheng H (2022) DRAMP 3.0: an enhanced comprehensive data repository of antimicrobial peptides. Nucleic Acids Res 50:D488–D496. https://doi.org/10.1093/nar/gkab651
Fan R, Yuan Y, Zhang Q, Zhou XR, Jia L, Liu Z, Yu C, Luo SZ, Chen L (2017) Isoleucine/leucine residues at “a” and “d” positions of a heptad repeat sequence are crucial for the cytolytic activity of a short anticancer lytic peptide. Amino Acids 49:193–202. https://doi.org/10.1007/s00726-016-2350-9
Acknowledgements
This work was supported by the Shanghai Natural Science Foundation of China Grant No.19ZR1461600 to AL.
Author information
Authors and Affiliations
Contributions
XY, GX, PS, ZG, ZF and BL drafted the manuscript cooperatively, ZG and AL prepared the figures, AL revised the manuscript, and YH, JY and JZ provided practical experience and suggestions for the biological application of AMPs. All the authors have read, commented on and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article did not contain any studies with animals performed by any of the authors.
Consent to Participate
All authors read and approved the final version of the manuscript.
Consent for Publication
All authors agree to publish this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ying, X., Xue, G., Sun, P. et al. Antimicrobial Peptides Targeting Streptococcus mutans: Current Research on Design, Screening and Efficacy. Curr Microbiol 81, 18 (2024). https://doi.org/10.1007/s00284-023-03540-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03540-5