Abstract
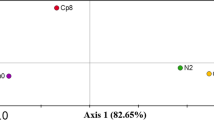
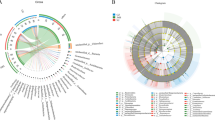
The last two decades have witnessed a large-scale conversion of crop cultivation areas into small and mid-sized tea plantations in Assam, India. Agricultural land-use pattern positively or negatively influences native hydrology and above- and belowground biodiversity. Very little is known about the effect of agricultural land-use patterns on the soil virus (especially, bacteriophage) community structure and function. This metagenomic-based study evaluated the rhizosphere viral community structure of three interlinked cultivation areas, viz., mixed cropping area (coded as CP1), tea-seed orchard (CP2), and monocropping tea cultivation (CP3). The bacteriophages belonged to four major classes with the dominance of Malgrandaviricetes (CP1: 79.37%; CP2: 64.62%; CP3: 4.85%) followed by Caudoviricetes (CP1: 20.49%; CP2: 35.22%; CP3: 90.29%), Faserviricetes (CP1: 0.03%; CP2: 0.08%; CP3: 3.88%), and Tectiliviricetes (CP1: 0.12%; CP2: 0.07%; CP3: 0.97%). Microviruses dominated the phage population in both CP1 and CP2, representing 79.35% and 64.59% of total bacteriophage abundance. Both CP1 and CP2 had higher bacteriophage richness (species richness, R in CP1: 65; R in CP2: 66) and lower evenness (Pielou's evenness index, J in CP1: 0.531; J in CP2: 0.579) compared to the CP3 (R: 30; J: 0.902). Principal component analysis of edaphic soil factors and bacteriophage community structure showed a reverse-proportional correlation between the levels of Al saturation, and exchangeable Al3+ ions with that of soil pH, and bacteriophage abundance. Our study indicates that monocropping tea cultivation soil bears less viral richness, abundance, and heterogeneity.



Similar content being viewed by others
Data Availability
The metagenomic sequence reads are available on the NCBI database with accession numbers PRJNA797555 (for the CP1 sample) and PRJNA698063 (for samples, CP2 and CP3). The MG-RAST Ids for the metagenomes are as follows: CP1: mgm4926379.3; CP2: mgm4916718.3; CP3: mgm4528392.3.
Code Availability
Not applicable.
References
Clokie MRJ, Millard AD, Letarov AV, Heaphy S (2011) Phages in nature. Bacteriophage 1:31–45. https://doi.org/10.4161/bact.1.1.14942
Koonin EV (2014) The origins of cellular life. Antonie Van Leeuwenhoek 106:27–41. https://doi.org/10.1007/s10482-014-0169-5
Howard-Varona C, Hargreaves KR, Abedon ST, Sullivan MB (2017) Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J 11:1511–1520. https://doi.org/10.1038/ismej.2017.16
Thingstad TF (2000) Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol Oceanogr 45:1320–1328. https://doi.org/10.4319/lo.2000.45.6.1320
Heinrichs ME, Tebbe DA, Wemheuer B et al (2020) Impact of viral lysis on the composition of bacterial communities and dissolved organic matter in deep-sea sediments. Viruses 12:922. https://doi.org/10.3390/v12090922
Suttle CA, Chan AM, Cottrell MT (1990) Infection of phytoplankton by viruses and reduction of primary productivity. Nature 347:467–469. https://doi.org/10.1038/347467a0
Brum JR, Sullivan MB (2015) Rising to the challenge: accelerated pace of discovery transforms marine virology. Nat Rev Microbiol 13:147–159. https://doi.org/10.1038/nrmicro3404
Weitz JS, Wilhelm SW (2012) Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol Rep. https://doi.org/10.3410/B4-17
Santos-Medellin C, Zinke LA, ter Horst AM et al (2021) Viromes outperform total metagenomes in revealing the spatiotemporal patterns of agricultural soil viral communities. ISME J 15:1956–1970. https://doi.org/10.1038/s41396-021-00897-y
Williamson KE, Fuhrmann JJ, Wommack KE, Radosevich M (2017) Viruses in soil ecosystems: an unknown quantity within an unexplored territory. Annu Rev Virol 4:201–219. https://doi.org/10.1146/annurev-virology-101416-041639
Buttimer C, McAuliffe O, Ross RP et al (2017) Bacteriophages and bacterial plant diseases. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00034
Kimura M, JIA Z-J, Nakayama N, Asakawa S (2008) Ecology of viruses in soils: past, present and future perspectives. Soil Sci Plant Nutr 54:1–32. https://doi.org/10.1111/j.1747-0765.2007.00197.x
Roy S (2011) Historical review of growth of tea industries in India: a study of Assam tea. Int Con Soc Sci Humanity 1:116–170
Sharma CK, Barua P (2017) Small tea plantation and its impact on the rural landscape of contemporary Assam. Int J Rural Manag 13:140–161. https://doi.org/10.1177/0973005217725454
Baruah P (2017) Wild teas of Assam and North East India. J Tea Sci Res. https://doi.org/10.5376/jtsr.2017.07.0007
Dikshit KR, Dikshit JK (eds) (2014) North-East India: Land, People and Economy. Springer Netherlands, Dordrecht
Bainard LD, Koch AM, Gordon AM, Klironomos JN (2013) Growth response of crops to soil microbial communities from conventional monocropping and tree-based intercropping systems. Plant Soil 363:345–356. https://doi.org/10.1007/s11104-012-1321-5
Borrelli P, Robinson DA, Panagos P et al (2020) Land use and climate change impacts on global soil erosion by water (2015–2070). Proc Natl Acad Sci 117:21994–22001. https://doi.org/10.1073/pnas.2001403117
Li S, Li H, Yang C et al (2016) Rates of soil acidification in tea plantations and possible causes. Agric Ecosyst Environ 233:60–66. https://doi.org/10.1016/j.agee.2016.08.036
Ding ZJ, Shi YZ, Li GX et al (2021) Tease out the future: How tea research might enable crop breeding for acid soil tolerance. Plant Commun 2:100182. https://doi.org/10.1016/j.xplc.2021.100182
Jaiswal SK, Naamala J, Dakora FD (2018) Nature and mechanisms of aluminium toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol Fertil Soils 54:309–318. https://doi.org/10.1007/s00374-018-1262-0
Bora SS, Dey KK, Borah M et al (2021) Niche differentiation of belowground microorganisms and their functional signatures in Assam type tea (Camellia sinensis var. assamica). Arch Microbiol 203:5661–5674. https://doi.org/10.1007/s00203-021-02547-5
Black CA (1965) Methods of Soil Analysis. American Society of Agronomy, Madison
Casanova M, Tapia E, Seguel O, Salazar O (2016) Direct measurement and prediction of bulk density on alluvial soils of central Chile. Chil J Agric Res 76:105–113. https://doi.org/10.4067/S0718-58392016000100015
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter, and proposed modification of the chromic acid tritation method. Soil Sci 37:29–38. https://doi.org/10.1097/00010694-193401000-00003
Ciesielski H, Sterckeman T, Santerne M, Willery J (1997) Determination of cation exchange capacity and exchangeable cations in soils by means of cobalt hexamine trichloride. Effects of experimental conditions. Agron EDP Sci 17:1–7
Shuman LM (1990) Comparison of exchangable Al, extractable Al, and Al in soil fractions. Can J Soil Sci 70:263–275. https://doi.org/10.4141/cjss90-028
Wilke A, Harrison T, Wilkening J et al (2012) The M5nr: a novel non-redundant database containing protein sequences and annotations from multiple sources and associated tools. BMC Bioinform 13:141. https://doi.org/10.1186/1471-2105-13-141
Pruitt KD, Tatusova T, Maglott DR (2007) NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 35:D61–D65. https://doi.org/10.1093/nar/gkl842
Hammer DAT, Ryan PD, Hammer Ø, Harper DAT (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Behera MD, Kushwaha SPS, Roy PS (2002) High plant endemism in an Indian hotspot – eastern Himalaya. Biodivers Conserv 11:669–682. https://doi.org/10.1023/A:1015596309833
Foster D, Swanson F, Aber J et al (2003) The importance of land-use legacies to ecology and conservation. Bioscience 53:77–88. https://doi.org/10.1641/0006-3568(2003)053[0077:TIOLUL]2.0.CO;2
Koskella B, Brockhurst MA (2014) Bacteria–phage coevolution as a driver of ecological and evolutionary processes in microbial communities. Fems Microbiol Rev 38:916. https://doi.org/10.1111/1574-6976.12072
Arber W (2014) Horizontal gene transfer among bacteria and its role in biological evolution. Life 4:217–224. https://doi.org/10.3390/life4020217
Keen EC (2014) Tradeoffs in bacteriophage life histories. Bacteriophage 4:e28365. https://doi.org/10.4161/bact.28365
Bevivino A, Paganin P, Bacci G et al (2014) Soil bacterial community response to differences in agricultural management along with seasonal changes in a mediterranean region. PLoS ONE 9:e105515. https://doi.org/10.1371/journal.pone.0105515
Goss-Souza D, Mendes LW, Borges CD et al (2019) Amazon forest-to-agriculture conversion alters rhizosphere microbiome composition while functions are kept. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiz009
Moore JC (2013) Diversity, Taxonomic versus Functional. In: Scheiner SM (ed) Encyclopedia of Biodiversity. Elsevier, pp 648–656
Han L-L, Yu D-T, Zhang L-M et al (2017) Genetic and functional diversity of ubiquitous DNA viruses in selected Chinese agricultural soils. Sci Rep 7:45142. https://doi.org/10.1038/srep45142
Khalifa AYZ, Alsyeeh A-M, Almalki MA, Saleh FA (2016) Characterization of the plant growth promoting bacterium, Enterobacter cloacae MSR1, isolated from roots of non-nodulating Medicago sativa. Saudi J Biol Sci 23:79–86. https://doi.org/10.1016/j.sjbs.2015.06.008
Roux S, Krupovic M, Poulet A et al (2012) Evolution and diversity of the microviridae viral family through a collection of 81 new complete genomes assembled from virome reads. PLoS ONE 7:e40418. https://doi.org/10.1371/journal.pone.0040418
Nwokolo NL, Enebe MC (2022) Shotgun metagenomics evaluation of soil fertilization effect on the rhizosphere viral community of maize plants. Antonie Van Leeuwenhoek 115:69–78. https://doi.org/10.1007/s10482-021-01679-4
Arafat Y, Ud Din I, Tayyab M et al (2020) Soil sickness in aged tea plantation is associated with a shift in microbial communities as a result of plant polyphenol accumulation in the tea gardens. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00601
Nordstrom DK, May HM (1996) Aqueous Equilibrium Data for Mononuclear Aluminum Species. In: Sposito G (ed) The Environmental Chemistry of Aluminum. CRC Press, pp 39–80
Bojórquez-Quintal E, Escalante-Magaña C, Echevarría-Machado I, Martínez-Estévez M (2017) Aluminum, a friend or foe of higher plants in acid soils. Front Plant Sci 8:1767
Abdul Rahman NSN, Abdul Hamid NW, Nadarajah K (2021) Effects of abiotic stress on soil microbiome. Int J Mol Sci 22:9036. https://doi.org/10.3390/ijms22169036
Miljaković D, Marinković J, Balešević-Tubić S (2020) The significance of bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8:1037. https://doi.org/10.3390/microorganisms8071037
Dastogeer KMG, Tumpa FH, Sultana A et al (2020) Plant microbiome–an account of the factors that shape community composition and diversity. Curr Plant Biol 23:100161. https://doi.org/10.1016/j.cpb.2020.100161
Quaiser A, Dufresne A, Ballaud F et al (2015) Diversity and comparative genomics of microviridae in sphagnum- dominated peatlands. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00375
Funding
This study did not receive any particular funding.
Author information
Authors and Affiliations
Contributions
MB and SSB contributed to conceptualization. SSB, RSN, and AC contributed to methodology. DJH, NRS, AD, and AC contributed to formal analysis and investigation. SSB contributed to writing—original draft preparation. MB and DJH contributed to writing—review and editing. MB contributed to funding acquisition and supervision. MG and MB contributed to resources.
Corresponding author
Ethics declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bora, S.S., Naorem, R.S., Hazarika, D.J. et al. Agricultural Land Use Influences Bacteriophage Community Diversity, Richness, and Heterogeneity. Curr Microbiol 80, 10 (2023). https://doi.org/10.1007/s00284-022-03129-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03129-4