Abstract
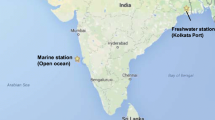
Inadvertent introductions of alien species via biofilms as a vector released through ballast water are of environmental importance, yet their consequences are not much known. In the present study, biofilm communities developed in an inland freshwater port under in situ and dark conditions were subjected to long-term dark incubations. Subsequently, the impact of these aged biofilms as vectors on estuarine water column communities were evaluated using microcosm experiments in the laboratory. Variations in biofilm and planktonic microbial communities were quantified using quantitative PCR.
Upon prolonged dark incubation, a shift in bacterial diversity with an increase in tolerant bacterial communities better adapted to stress was observed. Actinobacteria were the dominant taxa in both aged biofilms upon dark incubations. The laboratory studies indicated that on exposure of these biofilms to estuarine water, resuscitation of Vibrio alginolyticus, V. parahaemolyticus, and V. cholerae from a dormant state existing in these biofilms to culturable form was observed. Moreover, the results revealed that both the biofilm types can pose a threat to the environment, but the degree of risk can be attributed to the imbalance caused by significant changes in the surrounding estuarine microbial communities. Consequently, this may result in either proliferation or decline of some genera with different metabolic potential and resuscitation of pathogenic forms not present earlier, thereby influencing the ecology of the environment. Quantifying these effects in the field using biofilm metagenomes with an emphasis on virulent species and understanding traits that enable them to adapt to changing environments is a way forward.





Similar content being viewed by others
Data Availability
The data sets supporting the results of this article are included within the article.
References
Litchman E (2010) Invisible invaders: non-pathogenic invasive microbes in aquatic and terrestrial ecosystems. Ecol Lett 13:1560–1572
Khandeparker L, Anil AC (2017) Global concerns of ship’s ballast water mediated translocation of bacteria. In: Naik M, Dubey S (eds) Marine pollution and microbial remediation. Springer, Singapore
Cohen AN, Carlton JT (1998) Accelerating invasion rate in a highly invaded estuary. Science 279:555–558
Carlton J, Ruiz G, Byers J, Cangelosi A, Dobbs F, Grosholz E, Leung B, MacIsaac H, Wonham M (2011) Assessing the relationship between propagule pressure and invasion risk in ballast water. The National Academies Press, Washington, DC
Kragh KN, Hutchison JB, Melaugh G, Rodesney C, Roberts AE, Irie Y, Jensen PØ, Diggle SP, Allen RJ, Gordon V, Bjarnsholt T (2016) Role of multicellular aggregates in biofilm formation. MBio 7(2):e00237-e316
Melaugh G, Hutchison J, Kragh KN, Irie Y, Roberts AEL, Bjarnsholt T, Diggle SP, Gordon VD, Allen RJ (2016) Shaping the growth behaviour of bacterial aggregates in biofilms. PLoS ONE 11(3):e0149683
Srivastava A, Seo SH, Ko SR, Ahn CY, Oh HM (2018) Bioflocculation in natural and engineered systems: current perspectives. Crit Rev Biotechnol 38(8):1176–1194
Drake LA, Meyer AE, Forsberg RL, Baier RE, Doblin MA, Heinemann S, Johnson WP, Koch M, Rublee P, Dobbs FC (2005) Potential invasion of microorganisms and pathogens via interior hull fouling: biofilms inside ballast water tanks. Biol Invasions 7:969–982
Balzer M, Witt N, Flemming HC, Wingender J (2010) Faecal indicator bacteria in river biofilms. Water Sci Technol 61(5):1105–1111
Shikuma NJ, Hadfield MG (2010) Marine biofilms on submerged surfaces are a reservoir for Escherichia coli and Vibrio cholerae. Biofouling 26:39–46
Drake LA, Doblin MA, Dobbs FC (2007) Potential microbial bioinvasions via ships’ ballast water, sediment, and biofilm. Mar Pollut Bull 55:333–341
Keller RP, Drake JM, Drew MB, Lodge DM (2011) Linking environmental conditions and ship movements to estimate invasive species transport across the global shipping network. Divers Distrib 17:93–102
Paiva F, Barco A, Chen Y, Mirzajani A, Chan FT, Lauringson V, Baltazar-Soares M, Zhan A, Bailey SA, Javidpour J, Briski E (2018) Is salinity an obstacle for biological invasions? Global Change Biol 24:2708–2720
Crespo D, Solan M, Leston S, Pardal MA, Dolbeth M (2018) Ecological consequences of invasion across the freshwater–marine transition in a warming world. Ecol Evol 8:1807–1817
Hede N, Khandeparker L (2018) Influence of darkness and aging on marine and freshwater biofilm microbial communities using microcosm experiments. Microb Ecol 76:314–317
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, p 173
Pfeffer C, Oliver JD (2003) A comparison of thiosulphate-citrate-bile salts-sucrose (TCBS) agar and thiosulphate-chloride-iodide (TCI) agar for the isolation of Vibrio species from estuarine environments. Lett Appl Microbiol 36:150–151
Khandeparker L, Anil AC, Naik SD, Gaonkar CC (2015) Daily variations in pathogenic bacterial populations in a monsoon influenced tropical environment. Mar Pollut Bull 96:337–343
Khandeparker L, Eswaran R, Gardade L, Kuchi N, Mapari K, Naik SD, Anil AC (2017) Elucidation of the tidal influence on bacterial populations in a monsoon influenced estuary through simultaneous observations. Environ Monit Assess 189:41
De Gregoris TB, Aldred N, Clare AS, Burgess JG (2011) Improvement of phylum and class- specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods 86:351–356
Ashelford KE, Weightman AJ, Fry JC (2002) PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with the RDP-II database. Nucleic Acids Res 30:3481–3489
Clarke KR, Warwick RM (1994) Similarity-based testing for community pattern: the 2-way layout with no replication. Marine Biol 118:167–176
Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk HP, Clément C, Ouhdouch Y, van Wezel GP (2016) Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80(1):1–43
Neyland EB (2009) Bacteria in ballast water: the shipping industry’s contributions to the transport and distribution of microbial species in Texas. Texas AM University, College Station
Lacey J, Goodfellow M, Alderson G (1978) The genus Actinomadura Lechevalier and Lechevalier. Zentralbl Bakteriol Suppl 6:107–117
Glöckner FO, Zaichikov E, Belkova N, Denissova L, Pernthaler J, Pernthaler A, Amann R (2000) Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of Actinobacteria. Appl Environ Microbiol 66(11):5053–5065
Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, van Sinderen D (2007) Genomics of actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev 71(3):495–548
Hill P, Krištùfek V, Dijkhuizen L, Boddy C, Kroetsch D, van Elsas JD (2011) Land use intensity controls actinobacterial community structure. Microb Ecol 61(2):286–302
Colwell RR, Brayton PR, Grimes DU, Rosazk DB, Huq SA, Palmer LM (1985) Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Nature Biotechnol 3:817–820
Colwell RR (2000) Viable but nonculturable bacteria: a survival strategy. J Infect Chemother 6:121–125
Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S (2014) Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front Public Health 2:1–9
Sapp M, Wichels A, Gerdts G (2007) Impacts of cultivation of marine diatoms on the associated bacterial community. Appl Environ Microbiol 73:3117–3120
Guannel ML, Horner-Devine MC, Rocap G (2011) Bacterial community composition differs with species and toxigenicity of the diatom Pseudo-nitzschia. Aquat Microb Ecol 64:117–133
Amin SA, Parker MS, Armbrust EV (2012) Interactions between diatoms and bacteria. Microbiol Mol Biol R 76:667–684
Rehnstam-Holm AS, Godhe A, Härnström K, Raghunath P, Saravanan V, Collin B, Karunasagar I, Karunasagar I (2010) Association between phytoplankton and Vibrio spp. along the southwest coast of India: a mesocosm experiment. Aquat Microb Ecol 58:127–139
Buchan A, LeCleir GR, Gulvik CA, González JM (2014) Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nat Rev Microbiol 12:686–698
Khandeparker L, D’Costa PM, Anil AC, Sawant SS (2014) Interactions of bacteria with diatoms: influence on natural marine biofilms. Marine Ecol 35(2):233–248
Dang H, Lovell CR (2016) Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol R 80:91–138
Schleper C, Nicol GW (2010) Ammonia-oxidising archaea – physiology, ecology and evolutionin. Adv Microb Physiol 57:1–41
Damashek J, Francis CA (2018) Microbial nitrogen cycling in estuaries: from genes to ecosystem processes. Estuaries Coasts 41:626–660
Veettil VP, Abdulaziz A, Chekidhenkuzhiyil J, Ramkollath LK, Hamza FK, Kalam BK, Ravunnikutty MK, Nair S (2015) Bacterial domination over Archaea in ammonia oxidation in a monsoon-driven tropical estuary. Microb Ecol 69:544–553
Griffiths BS (1989) Enhanced nitrification in the presence of bacteriophagous protozoa. Soil Biol Biochem 21:1045–1051
Verhagen FJ, Duyts H, Laanbroek HJ (1993) Effects of grazing by flagellates on competition for ammonium between nitrifying and heterotrophic bacteria in soil columns. Appl Environ Microbiol 59:2099–2106
Pogue AJ, Gilbride KA (2007) Impact of protozoan grazing on nitrification and the ammonia-and nitrite-oxidizing bacterial communities in activated sludge. Can J Microbiol 53:559–571
Hiorns WD, Hastings RC, Head IM, McCarthy AJ, Saunders JR, Pickup RW, Hall GH (1995) Amplification of 16S ribosomal RNA genes of autotrophic ammonia-oxidising bacteria. Microbiol 141:2793–2800
Kahla-Nakbi AB, Besbes A, Chaieb K, Rouabhia M, Bakhrouf A (2007) Survival of Vibrio alginolyticus in seawater and retention of virulence of its starved cells. Marine Environ Res 64:469–478
Mishra A, Taneja N, Sharma M (2012) Viability kinetics, induction, resuscitation and quantitative real-time polymerase chain reaction analyses of viable but nonculturable Vibrio cholerae O1 in freshwater microcosm. J Appl Microbiol 112:945–953
Fernández-Delgado M, García-Amado MA, Contreras M, Incani RN, Chirinos H, Rojas H, Suárez P (2015) Survival, induction and resuscitation of Vibrio cholerae from the viable but non-culturable state in the Southern Caribbean Sea. Rev Inst Med Trop Sao Paulo 57:21–26
Lutz C, Erken M, Noorian P, Sun S, McDougald D (2013) Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front Microbiol 4:375
Khandeparker L, Eswaran R, Gardade L, Kuchi MK, Naik SD, Anil AC (2017) Elucidation of the tidal influence on bacterial populations in a monsoon influenced estuary through simultaneous observations. Environ Monit Assess 189:41
Khandeparker L, Hede N, Eswaran R, Usgaonkar A, Anil AC (2017) Microbial dynamics in a tropical monsoon influenced estuary: Elucidation through field observations and microcosm experiments on biofilms. J Exp Mar Bio Ecol 497:86–98
Taneja N, Mishra A, Batra N, Gupta P, Mahindroo J, Mohan B (2019) Inland cholera in freshwater environs of north India. Vaccine. https://doi.org/10.1016/j.vaccine.2019.06.038
Kostakioti M, Hadjifrangiskou M, Hultgren J (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the post antibiotic era. Cold Spring Harb Perspect Med 3:1–23
D’Souza F, Bruin A, Biersteker R, Donnelly G, Klijnstra J, Rentrop C, Willemsen P (2010) Bacterial assay for the rapid assessment of antifouling and fouling release properties of coatings and materials. J Ind Microbiol Biotechnol 37(4):363–370
Wagner K, Besemer K, Burns NR, Battin TJ, Bengtsson MM (2015) Light availability affects stream biofilm bacterial community composition and function, but not diversity. Environ Microbiol 17(12):5036–5047
Mc Eldowney S, Fletcher M (1988) Effect of pH, temperature and growth condition on the adhesion of a gliding bacterium and three non-gliding bacteria to polystyrene. Microbial Ecol 16:183–195
Pederson K (1990) Biofilm development on stainless steel and PVC surfaces in drinking water. Water Res 24:239–243
Acknowledgements
We are thankful to the Director, National Institute of Oceanography, for his support and encouragement. We gratefully acknowledge Dr A.C. Anil, Head of the Department, for his support. The first author acknowledges Jawaharlal Nehru Memorial Fund for the award of JNMF Scholarship. This is NIO contribution No. 6921.
Funding
This study was funded by the Ballast Water Management Program, India (Ministry of Shipping and DG shipping) (GAP 2429), and CSIR funded Ocean Finder Program (PSC 0105).
Author information
Authors and Affiliations
Contributions
NH: Conceptualization, methodology, formal analysis, investigation, writing—original draft, review & editing. LK: Conceptualization, methodology, validation, formal analysis, investigation, resources, writing—original draft, review & editing, supervision. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The authors state that the experiments were carried out under controlled conditions in the laboratory. All the aspects of the study were conducted in compliance with relevant Institutional biosafety and biosecurity protocols, and the manuscript has been approved by the Patents and Publication Committee of the Institute.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hede, N., Khandeparker, L. Ecological Impacts of Aged Freshwater Biofilms on Estuarine Microbial Communities Elucidated Through Microcosm Experiments: A Microbial Invasion Perspective. Curr Microbiol 79, 210 (2022). https://doi.org/10.1007/s00284-022-02903-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02903-8