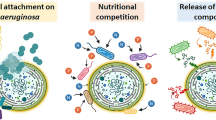
Abstract
A bacterium isolated from Lake Taihu was identified as Pseudomonas sp. A3CT, which performed different effects on Microcystis spp. Growth of Microcystis flos-aquae and Microcystis aeruginosa was assessed in co-culture with A3CT to determine the stimulatory or inhibitory effects on these toxic, bloom-forming Microcystis strains. Results demonstrated that the impacts of A3CT were species specific. A3CT promoted the growth of M. aeruginosa but inhibited growth of M. flos-aquae. To investigate the cause of this phenomenon, the chemical composition of A3CT exudates and the impact of exposure to A3CT exudates on the two Microcystis species were determined. Results suggested that the observed differential growth responses of the two microalgae to A3CT exposure might be related to two components in A3CT exudates NH4 + and cadaverine. Growth stimulation of M. aeruginosa by A3CT was significantly related to NH4 + concentration. Cadaverine possibly acted as a growth inhibitor of M. flos-aquae. The different effects of cadaverine on growth of the two Microcystis strains suggested that A3CT might play a role in intrageneric succession patterns observed during Microcystis blooms in Lake Taihu.


Similar content being viewed by others
References
Caron DA (1994) Inorganic nutrients, bacteria, and the microbial loop. Microb Ecol 28:295–298
Cassán F, Maiale S, Masciarelli O, Vidal A, Luna V, Ruiz O (2009) Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 45:12–19
Chaffin JD, Bridgeman TB, Heckathorn SA, Mishra S (2011) Assessment of Microcystis growth rate potential and nutrient status across a trophic gradient in western Lake Erie. J Gt Lakes Res 37:92–100
Chen Y, Qin B, Teubner K, Dokulil MT (2003) Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J Plankton Res 25:445–453
Clesceri LS (1998) Standard methods: for the examination of water and wastewater. American Public Health Ass, Washington
Cole JJ, Likens GE, Strayer DL (1982) Photosynthetically produced dissolved organic-carbon: an important carbon source for planktonic bacteria. Limnol Oceanogr 27:1080–1090
Delucca R, Mccracken MD (1977) Observations on interactions between naturally-collected bacteria and several species of algae. Hydrobiologia 55:71–75
Dominguez-Bocanegra AR, Legarreta IG, Jeronimo FM, Campocosio AT (2004) Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour Technol 92:209–214
Du J, Zhao G, Wang F, Zhao D, Chen X, Zhang S, Jia Y, Tian X (2013) Growth stimulation of Microcystis aeruginosa by a bacterium from hyper-eutrophic water (Taihu Lake, China). Aquat Ecol 47(3):303–313
Fuell C, Elliott KA, Hanfrey CC, Franceschetti M, Michael AJ (2010) Polyamine biosynthetic diversity in plants and algae. Plant Physiol Biochem 48:513–520
Han GM, Feng XG, Jia Y, Wang CY, He XB, Zhou QY, Tian XJ (2011) Isolation and evaluation of terrestrial fungi with algicidal ability from Zijin Mountain, Nanjing, China. J Microbiol 49:562–567
Hehmann A, Kaya K, Watanabe MM (2002) Selective control of Microcystis using an amino acid-a laboratory assay. J Appl Phycol 14:85–89
Hou HS, Wu CY (1998) Advance in research on plant hormones in algae. Studia Marina Sinica (Chin J) 40:167–176
Hua XH, Li JH, Li JJ, Zhang LH, Cui Y (2009) Selective inhibition of the cyanobacterium, Microcystis, by a Streptomyces sp. Biotechnol Lett 31:1531–1535
Hwang DF, Lu YH, Noguchi T (2003) Effects of exogenous polyamines on growth, toxicity, and toxin profile of Dinoflagellate Alexandrium minutum. Shokuhin Eiseigaku Zasshi 44:49–53
Imai H, Chang KH, Kusaba M, Nakano S (2009) Temperature-dependent dominance of Microcystis (Cyanophyceae) species: M. aeruginosa and M. wesenbergii. J Plankton Res 31:171–178
Jia Y, Han GM, Wang CY, Guo P, Jiang WX, Li XN, Tian XJ (2010) The efficacy and mechanisms of fungal suppression of freshwater harmful algal bloom species. J Hazard Mater 183:176–181
Kaya K, Liu YD, Shen YW, Xiao BD, Sano T (2005) Selective control of toxic Microcystis water blooms using lysine and malonic acid: an enclosure experiment. Environ Toxicol 20:170–178
Kemp A, John J (2006) Microcystins associated with Microcystis dominated blooms in the Southwest wetlands, Western Australia. Environ Toxicol 21:125–130
Krienitz L, Ballot A, Wiegand C, Kotut K, Codd GA, Pflugmacher S (2002) Cyanotoxin-producing bloom of Anabaena flos-aquae, Anabaena discoidea and Microcystis aeruginosa (Cyanobacteria) in Nyanza Gulf of Lake Victoria, Kenya. J Appl Bot 76:179–183
Lee YK, Ahn CY, Kim HS, Oh HM (2010) Cyanobactericidal effect of Rhodococcus sp. isolated from eutrophic lake on Microcystis sp. Biotechnol Lett 32:1673–1678
Lloret SM, Legua CM, Falco PC (2002) Preconcentration and dansylation of aliphatic amines using C18 solid-phase packings: application to the screening analysis in environmental water samples. J Chromatogr A 978:59–69
Maestrini ST, Balode M, Bechemin C, Purina I (1999) Nitrogenous organic substances as potential nitrogen sources, for summer phytoplankton in the gulf of Riga, eastern Baltic Sea. Plankton Biol Ecol 46:8–17
McCarthy MJ, Lavrentyev PJ, Yang LY, Zhang L, Chen YW, Qin BQ, Gardner WS (2007) Nitrogen dynamics and microbial food web structure during a summer cyanobacterial bloom in a subtropical, shallow, well-mixed, eutrophic lake (Lake Taihu, China). Hydrobiologia 581:195–207
Miller DL, Rodwell VW (1971) Metabolism of basic amino acids in Pseudomonas putida: intermediates in l-arginine catabolism. J Biol Chem 246:5053–5058
Nishibori N, Yuasa A, Sakai M, Fujihara S, Nishio S (2001) Free polyamine concentrations in coastal seawater during phytoplankton bloom. Fish Sci 67:79–83
Paerl HW, Huisman J (2008) Blooms like it hot. Science 320:57–58
Qian Z, Xia X, Lee SY (2011) Metabolic engineering of Escherichia coli for the production of cadaverine: a five carbon diamine. Biotechnol Bioeng 108:93–103
Rippka R, Desrulles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignment strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Rückert GV, Giani A (2004) Effect of nitrate and ammonium on the growth and protein concentration of Microcystis viridis Lemmermann (Cyanobacteria). Revista Brasileira de Botanica 27:325–331
Environmental monitoring systems lab (1993) USEPA methods for the determination of metals in environmental samples. Environmental monitoring systems lab, Cincinnati
Van der Westhuizen AJ, Eloff JN (1985) Effect of temperature and light on the toxicity and growth of the blue-green-alga Microcystis-aeruginosa (UV-006). Planta 163:55–59
Vickerman MM, Brossard KA, Funk DB, Jesionowski AM, Gill SR (2007) Phylogenetic analysis of bacterial and archaeal species in symptomatic and asymptomatic endodontic infections. J Med Microbiol 56:110–118
Virtanen AI, Lainc T (1937) The decarboxylation of l-tysine and l-aspartic acid. Enzymologia 3:266–270
Wu ZX, Shi JQ, Li RH (2009) Comparative studies on photosynthesis and phosphate metabolism of Cylindrospermopsis raciborskii with Microcystis aeruginosa and Aphanizomenon flos-aquae. Harmful Algae 8:910–915
Xu Y (2011) The ecological research on the Microcystis bloom in eutrophic water bodies, Nanjing Normal University, Nanjing
Yang Z, Geng LL, Wang W, Zhang J (2012) Combined effects of temperature, light intensity, and nitrogen concentration on the growth and polysaccharide content of Microcystis aeruginosa in batch culture. Biochem Syst Ecol 41:130–135
Zhai HL, Yang XQ, Hao SX, Cen JW, Wei Y, Shi H (2011) Optimization of operating conditions for HPLC determination of biogenic amines. Food Sci (Chin J) 32:180–184
Zhao GY, Du JJ, Jia Y, Lv YN, Han GM, Tian XJ (2012) The importance of bacteria in promoting algal growth in eutrophic lakes with limited available phosphorus. Ecol Eng 42:107–111
Zillen L, Conley DJ (2010) Hypoxia and cyanobacteria blooms: are they really natural features of the late Holocene history of the Baltic Sea? Biogeosciences 7:2567–2580
Acknowledgments
This work was financially supported by the Major Science and Technology Program for Water Pollution Control and Treatment (No. 2012ZX07204-004-003) and Ph.D. Programs Foundation of Ministry of Education of China (No. 20110091110018). We thank Dr. Nathan S Hall, University of North Carolina at Chapel Hill-Institute of Marine Sciences, for his valuable comments and suggestions during manuscript preparation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Du, J., Cheng, S., Shao, C. et al. Dual Roles of Cadaverine-Producing Pseudomonas sp. on Microcystis spp. in Hyper-Eutrophic Water. Curr Microbiol 69, 25–31 (2014). https://doi.org/10.1007/s00284-014-0544-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0544-5