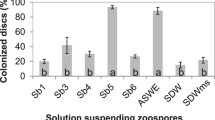
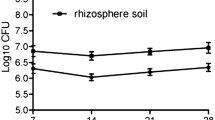
Abstract
This article correlates colonization with parameters, such as chemotaxis, biofilm formation, and bacterial growth, that are believed to be connected. We show here, by using two varieties of soybean plants that seeds axenically produced exudates, induced a chemotactic response in Bacillus amyloliquefaciens, whereas root exudates did not, even when the exudates, also collected under axenic conditions, were concentrated up to 200-fold. Root exudates did not support bacterial cell division, whereas seed exudates contain compounds that support active cell division and high cell biomass at stationary phase. Seed exudates of the two soybean varieties also induced biofilm formation. B. amyloliquefaciens colonized both seeds and roots, and plant variety significantly affected bacterial root colonization, whereas it did not affect seed colonization. Colonization of roots in B. amyloliquefaciens occurred despite the lack of chemotaxis and growth stimulation by root exudates. The data presented in this article suggest that soybean seed colonization, but not root colonization, by B. amyloliquefaciens is influenced by chemotaxis, growth, and biofilm formation and that this may be caused by qualitative changes of the composition of root exudates.



Similar content being viewed by others
References
Adler J (1973) A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol 74:77–91
Ali FS, Loynachan TE (1990) Inhibition of Bradyrhizobium japonicum by diffusates from soybean seed. Soil Biol Biochem 22:973–976
Bacilio-Jiménez M, Aguilar-Flores S, Ventura-Zapata E, Pérez-Campos E, Bouquelet S, Zenteno E (2003) Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil 249:271–277
Bais HP, Ray F, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants. Annu Rev Plant Biol 57:233–266
Barbour MW, Hattermann DR, Stacey G (1991) Chemotaxis of Bradyrhizobium japonicum to soybean exudates. Appl Environ Microbiol 57:2635–2639
Branda SS, Chu F, Kearns DB, Losick R, Kolter R (2006) A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238
Caetano-Anollés G, Wall LG, De Micheli AT, Macchi EM, Bauer WD, Favelukes G (1988) Role of motility and chemotaxis in efficiency of nodulation by Rhizobium melliloti. Plant Physiol 86:1228–1235
Chin-A-Woeng TFC, Bloemberg GV, Mulders IHM, Dekkers LC, Lugtenberg BJJ (2000) Root colonization is essential for biocontrol of tomato foot and root rot by the phenazine-1-carboxamide-producing bacterium Pseudomonas chlororaphis PCL1391. Mol Plant Microbe Interact 13:1340–1345
Currier WW, Strobel GA (1976) Chemotaxis of Rhizobium spp. to plant root exudates. Plant Physiol 57:820–823
de Weert S, Vermeiren H, Mulders I, Kuiper I, Hendrickx N, Bloemberg GV et al (2002) Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol Plant Microbe Interact 15:1173–1180
Hamon MA, Lazazzera BA (2001) The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol 42:1199–1209
Hiroyuki F, Masao S, Hidenori O, Yasufumi U, Tadayoshi S, Tatsuhiko M (1998) Chemotactic response to amino acids of fluorescent pseudomonas isolated from spinach roots grown in soils with different salinity levels. Soil Sci Plant Nutr 44:1–7
Kadouri D, Jurkevitch E, Okon Y (2003) Involvement of the reserve material poly-ß-hydroxybutirate in Azospirillum brasilense stress endurance and root colonization. Appl Environ Microbiol 69:3244–3250
Kamilova F, Validov S, Azarova T, Mulders I, Lugtenberg BJJ (2005) Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ Microbiol 7:1809–1817
Kato K, Arima Y (2006) Potential of seed and root exudates of the common bean Phaseolus vulgaris L. for immediate induction of rhizobial chemotaxis and nod genes. Soil Sci Plant Nutr 52:432–437
Kuzyakov Y, Raskatov A, Kaupenjohann M (2003) Turnover and distribution of root exudates of Zea mays. Plant Soil 254:317–327
Landa BB, Hervás A, Bettiol W, Jiménez-Díaz RM (1997) Antagonistic activity of bacteria from the chickpea rhizosphere against Fusarium oxysporum f. sp. ciceris. Phytoparasitica 25:305–318
Lorda GS, Balatti AP (1996) Designing media I. Production of high cell concentrations of Rhizobium and Bradyrhizobium. In: Balatti AP, Freire, JRJ (eds) Legume inoculants. Selection and characterization of strains, production, use and management. Editorial Kingraf, Buenos Aires, Argentina, pp 78–93
Lugtenberg BJJ, Dekkers LC (1999) What make Pseudomonas bacteria rhizosphere competent? Environ Microbiol 1:9–13
Lugtenberg BJJ, Kravchenko LV, Simons M (1999) Tomato seed and root exudate sugars: composition, utilization by Pseudomonas biocontrol strains, and role in rhizosphere colonization. Environ Microbiol 1:439–446
Lugtenberg BJJ, Dekkers LC, Bloemberg GV (2002) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39:461–490
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10
Molina MA, Ramos JL, Espinosa-Urgel M (2003) Plant-associated biofilms. Rev Environ Sci Biotechnol 2:99–108
Morris CE, Monier JM (2003) The ecological significance of biofilm formation by plant-associated bacteria. Annu Rev Phytopathol 41:429–453
Mozafar A, Duss F, Oertli JJ (1992) Effect of Pseudomonas fluorescens on the root exudates of two tomato mutans differently sensitive to Fe chlorosis. Plant Soil 144:167–176
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tissue culture. Physiol Plant 15:473–497
Niu C, Gilbert ES (2004) Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl Environ Microbiol 70:6951–6956
Ordal G, Goldman D (1975) Chemotaxis away from uncouplers of oxidative phosphorylation in Bacillus subtilis. Science 189:802–805
Ordal G, Katharine G (1977) Chemotaxis toward amino acids by Bacillus subtilis. J Bacteriol 129:151–155
Phillips DA, Tama CF, King MD, Bhuvaneswari TV, Teuber LR (2004) Microbial products trigger amino acid exudation from plant roots. Plant Physiol 136:2887–2894
Schippers B, Baker AW, Bakker PAHM (1987) Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Annu Rev Phytopathol 25:339–358
Sharma M, Anand SK (2002) Swarming: a coordinated bacterial activity. Current Sci 83:707–715
Somasegaran P, Hoben HJ (1994) Handbook for rhizobia. Springer-Verlag, New York, NY, p 337–341
Stanley NR, Britton RA, Grossman AD, Lazazzera BA (2003) Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J Bacteriol 185:1951–1957
Thomashow LS (1996) Biological control of plant root pathogens. Curr Opin Biotechnol 7:343–347
Vande Broek A, Lambrecht M, Vanderleyden J (1998) Bacterial chemotactic motility is important for the initiation of wheat root colonization by Azospirillum brasilense. Microbiology 144:2599–2606
Zheng XY, Sinclair JB (1996) Chemotactic response of Bacillus megaterium strain B153-2-2 to soybean root and seed exudates. Physiol Mol Plant Pathol 48:21–35
Acknowledgments
The authors acknowledge financial support received from the Agencia Nacional de Promoción Científica y Tecnológica (Grant No. PICT 01-10892); the Centro Argentino-Brasileño de Biotecnología (Grant No. 13AR-07BR); and the Consejo Nacional de Investigaciones Científicas y Técnicas (Grant No. PIP 5003). We thank F. del Norte for kindly supplying the seeds and the phenotypic characteristics of the soybean varieties used here.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yaryura, P.M., León, M., Correa, O.S. et al. Assessment of the Role of Chemotaxis and Biofilm Formation as Requirements for Colonization of Roots and Seeds of Soybean Plants by Bacillus amyloliquefaciens BNM339. Curr Microbiol 56, 625–632 (2008). https://doi.org/10.1007/s00284-008-9137-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9137-5