Abstract
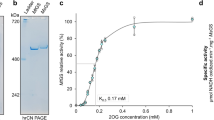
A L-methionine-D,L-sulfoximine-resistant mutant of the cyanobacterium Anabaena variabilis, strain SA1, excreted the ammonium ion generated from N2 reduction. In order to determine the biochemical basis for the NH4 +-excretion phenotype, glutamine synthetase (GS) was purified from both the parent strain SA0 and from the mutant. GS from strain SA0 (SA0-GS) had a pH optimum of 7.5, while the pH optimum for GS from strain SA1 (SA1-GS) was 6.8. SA1-GS required Mn+2 for optimum activity, while SA0-GS was Mg+2 dependent. SA0-GS had the following apparent K m values at pH 7.5: glutamate, 1.7 mM; NH4 +, 0.015 mM; ATP, 0.13 mM. The apparent K m for substrates was significantly higher for SA1-GS at its optimum pH (glutamate, 9.2 mM; NH4 +, 12.4 mM; ATP, 0.17 mM). The amino acids alanine, aspartate, cystine, glycine, and serine inhibited SA1-GS less severely than the SA0-GS. The nucleotide sequences of glnA (encoding glutamine synthetase) from strains SA0 and SA1 were identical except for a single nucleotide substitution that resulted in a Y183C mutation in SA1-GS. The kinetic properties of SA1-GS isolated from E. coli or Klebsiella oxytoca glnA mutants carrying the A. variabilis SA1 glnA gene were also similar to SA1-GS isolated from A. variabilis strain SA1. These results show that the NH4 +-excretion phenotype of A. variabilis strain SA1 is a direct consequence of structural changes in SA1-GS induced by the Y183C mutation, which elevated the K m values for NH4 + and glutamate, and thus limited the assimilation of NH4 + generated by N2 reduction. These properties and the altered divalent cation-mediated stability of A. variabilis SA1-GS demonstrate the importance of Y183 for NH4 + binding and metal ion coordination.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 3 July 2002 / Accepted: 29 July 2002
Rights and permissions
About this article
Cite this article
Healy, F., Latorre, C., Albrecht, S. et al. Altered Kinetic Properties of Tyrosine-183 to Cysteine Mutation in Glutamine Synthetase of Anabaena variabilis Strain SA1 Is Responsible for Excretion of Ammonium Ion Produced by Nitrogenase. Curr Microbiol 46, 0423–0431 (2003). https://doi.org/10.1007/s00284-002-3914-3
Issue Date:
DOI: https://doi.org/10.1007/s00284-002-3914-3