Abstract
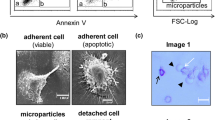
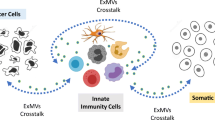
Complement-mediated cell death is caused by C5b-9, the membrane attack complex (MAC) composed of the five complement proteins C5b, C6, C7, C8, and C9. Assembly of the C5b-9 complex initiates oligomerization of C9 and production of a transmembrane protein channel that inflicts damage to target cells. For protection, cells eliminate the MAC from their surface either by ectocytosis (direct emission of membrane vesicles) or by endocytosis (internalization). The process of ectosome release is rapid and involves cytosolic Ca2+ and activation of protein kinases, such as protein kinase C (PKC) and extracellular signal-regulated protein kinase (ERK). Recently, the involvement of mortalin (also known as GRP75 and mitochondrial hsp70) in MAC elimination has been suggested. Extracellular application of antibodies directed to mortalin increases cell sensitivity to MAC-mediated lysis. Release of membrane vesicles is ubiquitous and enhanced in apoptotic or tumor cells and upon cell activation. Composition of the ectosomes (also often referred to as microparticles) membrane proteins and lipids appears to be different from those of the original plasma membrane, indicating involvement of a selective sorting process during ectosome formation. Exosomes (unlike ectosomes) are membrane vesicles generated by endocytosis, endosome sorting into perinuclear multivesicular bodies (MVB) and exocytosis of MVBs. Exosomes appear to be different in size and composition from ectosomes. Exosome-associated MAC has also been described. Although research on ectosomes and exosomes is still limited, physiological roles in coagulation, vascular functions, angiogenesis, wound healing and development have been attributed to these shed membrane vesicles. On the other hand, there are indications that elevated levels of ectosomes and exosomes may predispose to morbidity. Membrane vesicles released by cells exposed to complement MAC may play roles in health and disease beyond protection from cell death.


Similar content being viewed by others
Abbreviations
- ERK:
-
Extracellular signal-regulated protein kinase
- fMLP:
-
Formyl-methionyl-leucyl-phenylalanine
- GPI:
-
Glycosyl-phosphatidylinositol
- GRP75:
-
75-kDa glucose-regulated protein
- MAC:
-
Membrane attack complex of complement
- MVB:
-
Endocytic multivesicular bodies
- PKC:
-
Protein kinase C
References
Ahn YS, Jy W, Jimenez JJ et al (2004) More on: cellular microparticles: what are they bad or good for? J Thromb Haemost 2:1215
Anderson HC (2003) Matrix vesicles and calcification. Curr Rheumatol Rep 5:222
Andre F, Escudier B, Angevin E et al (2004) Exosomes for cancer immunotherapy. Ann Oncol 15(Suppl 4):iv141
Arvidson G, Ronquist G, Wikander G et al (1989) Human prostasome membranes exhibit very high cholesterol/phospholipid ratios yielding high molecular ordering. Biochim Biophys Acta 984:167
Bohana-Kashtan O, Pinna LA, Fishelson Z (2005) Extracellular phosphorylation of C9 by protein kinase CK2 regulates complement-mediated lysis. Eur J Immunol 35:1939
Bohana-Kashtan O, Ziporen L, Donin N et al (2004) Cell signals transduced by complement. Mol Immunol 41:583
Butikofer P, Kuypers FA, Xu CM et al (1989) Enrichment of two glycosyl-phosphatidylinositol-anchored proteins, acetylcholinesterase and decay accelerating factor, in vesicles released from human red blood cells. Blood 74:1481
Campbell AK, Daw RA, Hallett MB et al (1981) Direct measurement of the increase in intracellular free calcium ion concentration in response to the action of complement. Biochem J 194:551
Campbell AK, Daw RA, Luzio JP (1979) Rapid increase in intracellular free Ca2+ induced by antibody plus complement. FEBS Lett 107:55
Campbell AK, Morgan BP (1985) Monoclonal antibodies demonstrate protection of polymorphonuclear leukocytes against complement attack. Nature 317:164
Carette J, Lehnert S, Chow TY (2002) Implication of PBP74/mortalin/GRP75 in the radio-adaptive response. Int J Radiat Biol 78:183
Carney DF, Hammer CH, Shin ML (1986) Elimination of terminal complement complexes in the plasma membrane of nucleated cells: influence of extracellular Ca2+ and association with cellular Ca2+. J Immunol 137:263
Carney DF, Koski CL, Shin ML (1985) Elimination of terminal complement intermediates from the plasma membrane of nucleated cells: the rate of disappearance differs for cells carrying C5b-7 or C5b-8 or a mixture of C5b-8 with a limited number of C5b-9. J Immunol 134:1804
Carney DF, Lang TJ, Shin ML (1990) Multiple signal messengers generated by terminal complement complexes and their role in terminal complement complex elimination. J Immunol 145:623
Clayton A, Harris CL, Court J et al (2003) Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol 33:522
Cybulsky AV, Bonventre JV, Quigg RJ et al (1990) Cytosolic calcium and protein kinase C reduce complement-mediated glomerular epithelial injury. Kidney Int 38:803
Dolo V, D'Ascenzo S, Violini S et al (1999) Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis 17:131
Dundas SR, Lawrie LC, Rooney PH et al (2005) Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol 205:74
Dworniczak B, Mirault ME (1987) Structure and expression of a human gene coding for a 71 kd heat shock ‘cognate’ protein. Nucleic Acids Res 15:5181
Fevrier B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16:415
Fishelson Z, Attali G, Mevorach D (2001a) Complement and apoptosis. Mol Immunol 38:207
Fishelson Z, Hochman I, Greene LE et al (2001b) Contribution of heat shock proteins to cell protection from complement-mediated lysis. Int Immunol 13:983
Fishelson Z, Kopf E, Paas Y et al (1989) Protein phosphorylation as a mechanism of resistance against complement damage. Prog Immunol 7:205
Frade R (1999) Structure and functions of proteases which cleave human C3 and are expressed on normal or tumor human cells: some are involved in tumorigenic and metastatic properties of human melanoma cells. Immunopharmacology 42:39
Freyssinet JM (2003) Cellular microparticles: what are they bad or good for? J Thromb Haemost 1:1655
Gasser O, Hess C, Miot S et al (2003) Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res 285:243
Gasser O, Schifferli JA (2004) Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104:2543
Gasser O, Schifferli JA (2005) Microparticles released by human neutrophils adhere to erythrocytes in the presence of complement. Exp Cell Res 307:381. DOI 10.116/j.yexcr.2005.03.011
Gilbert GE, Sims PJ, Wiedmer T et al (1991) Platelet-derived microparticles express high affinity receptors for factor VIII. J Biol Chem 266:17261
Ginestra A, Miceli D, Dolo V et al (1999) Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res 19:3439
Graves LE, Ariztia EV, Navari JR et al (2004) Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res 64:7045
Hakulinen J, Junnikkala S, Sorsa T et al (2004) Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur J Immunol 34:2620
Halperin JA, Taratuska A, Nicholson-Weller A (1993) Terminal complement complex C5b-9 stimulates mitogenesis in 3T3 cells. J Clin Invest 91:1974
Heijnen HF, Schiel AE, Fijnheer R et al (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94:3791
Hess C, Sadallah S, Hefti A et al (1999) Ectosomes released by human neutrophils are specialized functional units. J Immunol 163:4564
Iida K, Whitlow MB, Nussenzweig V (1991) Membrane vesiculation protects erythrocytes from destruction by complement. J Immunol 147:2638
Jurianz K, Ziegler S, Donin N et al (2001) K562 erythroleukemic cells are equipped with multiple mechanisms of resistance to lysis by complement. Int J Cancer 93:848
Jurianz K, Ziegler S, Garcia-Schuler H et al (1999) Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol 36:929
Kerjaschki D, Schulze M, Binder S et al (1989) Transcellular transport and membrane insertion of the C5b-9 membrane attack complex of complement by glomerular epithelial cells in experimental membranous nephropathy. J Immunol 143:546
Kilgore KS, Schmid E, Shanley TP et al (1997) Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am J Pathol 150:2019
Kim CW, Lee HM, Lee TH et al (2002) Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res 62:6312
Kim HT, Nelson EL, Clayberger C et al (1995) Gamma delta T cell recognition of tumor Ig peptide. J Immunol 154:1614
Kojima A, Iwata K, Seya T et al (1993) Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol 151:1519
Koski CL, Ramm LE, Hammer CH et al (1983) Cytolysis of nucleated cells by complement: cell death displays multi-hit characteristics. Proc Natl Acad Sci U S A 80:3816
Kraus S, Fishelson Z (2000) Cell desensitization by sublytic C5b-9 complexes and calcium ionophores depends on activation of protein kinase C. Eur J Immunol 30:1272
Kraus S, Seger R, Fishelson Z (2001) Involvement of the ERK mitogen-activated protein kinase in cell resistance to complement-mediated lysis. Clin Exp Immunol 123:366
Liu D, Liu F, Song YK (1995) Recognition and clearance of liposomes containing phosphatidylserine are mediated by serum opsonin. Biochim Biophys Acta 1235:140
Llorente A, de Marco MC, Alonso MA (2004) Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J Cell Sci 117:5343
Lutz HU, Liu SC, Palek J (1977) Release of spectrin-free vesicles from human erythrocytes during ATP depletion. I. Characterization of spectrin-free vesicles. J Cell Biol 73:548
Mack M, Kleinschmidt A, Bruhl H et al (2000) Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med 6:769
MacKenzie A, Wilson HL, Kiss-Toth E et al (2001) Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15:825
Martin S, Tesse A, Hugel B et al (2004) Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation 109:1653
Martinez MC, Tesse A, Zobairi F et al (2005) Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol 288:H1004
Mayer MM (1961) On the destruction of erythrocytes and other cells by antibody and complement. Cancer Res 21:1262
Meri S, Morgan BP, Davies A et al (1990) Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology 71:1
Morel O, Toti F, Hugel B et al (2004) Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol 11:156
Morgan BP (1989) Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J 264:1
Morgan BP, Campbell AK (1985) The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J 231:205
Morgan BP, Dankert JR, Esser AF (1987) Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol 138:246
Morgan BP, Imagawa DK, Dankert JR et al (1986) Complement lysis of U937, a nucleated mammalian cell line in the absence of C9: effect of C9 on C5b-8 mediated cell lysis. J Immunol 136:3402
Morgan BP, Luzio JP, Campbell AK (1986) Intracellular Ca2+ and cell injury: a paradoxical role of Ca2+ in complement membrane attack. Cell Calcium 7:399
Muller-Eberhard HJ (1986) The membrane attack complex of complement. Annu Rev Immunol 4:503
Nauta AJ, Daha MR, Tijsma O et al (2002) The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol 32:783
Nicholson-Weller A, Wang CE (1994) Structure and function of decay accelerating factor CD55. J Lab Clin Med 123:485
Niculescu F, Badea T, Rus H (1999) Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis 142:47
Niculescu F, Rus H, Shin S et al (1993) Generation of diacylglycerol and ceramide during homologous complement activation. J Immunol 150:214
Nusbaum P, Laine C, Bouaouina M et al (2005) Distinct signaling pathways are involved in leukosialin (CD43) down-regulation, membrane blebbing, and phospholipid scrambling during neutrophil apoptosis. J Biol Chem 280:5843
Ohanian SH, Schlager SI, Borsos T (1978) Molecular interactions of cells with antibody and complement: influence of metabolic and physical properties of the target on the outcome of humoral immune attack. Contemp Topics Mol Immunol 7:153
Paas Y, Bohana-Kashtan O, Fishelson Z (1999) Phosphorylation of the complement component, C9, by an ecto-protein kinase of human leukemic cells. Immunopharmacology 42:175
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33:967
Papadimitriou JC, Drachenberg CB, Shin ML et al (1994) Ultrastructural studies of complement mediated cell death: a biological reaction model to plasma membrane injury. Virchows Arch 424:677
Pascual M, Lutz HU, Steiger G et al (1993) Release of vesicles enriched in complement receptor 1 from human erythrocytes. J Immunol 151:397
Pilzer D, Fishelson Z (2005) Mortalin/GRP75 promotes release of membrane vesicles from immune attacked cells and protection from complement-mediated lysis. Int Immunol 17:1239
Ramm LE, Whitlow MB, Koski CL et al (1983) Elimination of complement channels from the plasma membranes of U937, a nucleated mammalian cell line: temperature dependence of the elimination rate. J Immunol 131:1411
Ran Q, Wadhwa R, Kawai R et al (2000) Extramitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem Biophys Res Commun 275:174
Reiter Y, Ciobotariu A, Fishelson Z (1992) Sublytic complement attack protects tumor cells from lytic doses of antibody and complement. Eur J Immunol 22:1207
Riteau B, Faure F, Menier C et al (2003) Exosomes bearing HLA-G are released by melanoma cells. Hum Immunol 64:1064
Ronquist G, Brody I (1985) The prostasome: its secretion and function in man. Biochim Biophys Acta 822:203
Rus HG, Niculescu F, Shin ML (1996) Sublytic complement attack induces cell cycle in oligodendrocytes. J Immunol 156:4892
Sabatier F, Roux V, Anfosso F et al (2002) Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 99:3962
Satta N, Toti F, Feugeas O et al (1994) Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol 153:3245
Scolding NJ, Morgan BP, Houston WA et al (1989) Vesicular removal by oligodendrocytes of membrane attack complexes formed by activated complement. Nature 339:620
Shimizu A, Masuda Y, Kitamura H et al (2000) Complement-mediated killing of mesangial cells in experimental glomerulonephritis: cell death by a combination of apoptosis and necrosis. Nephron 86:152
Shirazi Y, McMorris FA, Shin ML (1989) Arachidonic acid mobilization and phosphoinositide turnover by the terminal complement complex, C5b-9, in rat oligodendrocyte x C6 glioma cell hybrids. J Immunol 142:4385
Sims PJ, Faioni EM, Wiedmer T et al (1988) Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem 263:18205
Skokos D, Botros HG, Demeure C et al (2003) Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 170:3037
Soane L, Cho HJ, Niculescu F et al (2001) C5b-9 terminal complement complex protects oligodendrocytes from death by regulating Bad through phosphatidylinositol 3-kinase/Akt pathway. J Immunol 167:2305
Sreedhar AS, Mihaly K, Pato B et al (2003) Hsp90 inhibition accelerates cell lysis. Anti-Hsp90 ribozyme reveals a complex mechanism of Hsp90 inhibitors involving both superoxide- and Hsp90-dependent events. J Biol Chem 278:35231
Stein JM, Luzio JP (1991) Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J 274(Pt 2):381
Takano S, Wadhwa R, Yoshii Y et al (1997) Elevated levels of mortalin expression in human brain tumors. Exp Cell Res 237:38
Taylor DD, Gercel-Taylor C (2005) Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 92:305
Tedesco F, Pausa M, Nardon E et al (1997) The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med 185:1619
Thery C, Duban L, Segura E et al (2002) Indirect activation of naive CD4+T cells by dendritic cell-derived exosomes. Nat Immunol 3:1156
Tschopp J, Podack ER, Muller-Eberhard HJ (1982) Ultrastructure of the membrane attack complex of complement: detection of the tetramolecular C9-polymerizing complex C5b-8. Proc Natl Acad Sci U S A 79:7474
van Blitterswijk WJ, Emmelot P, Hilkmann HA et al (1979) Rigid plasma-membrane-derived vesicles, enriched in tumour-associated surface antigens (MLr), occurring in the ascites fluid of a murine leukaemia (GRSL). Int J Cancer 23:62
Van Niel G, Mallegol J, Bevilacqua C et al (2003) Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 52:1690
Vanbuskirk A, Crump BL, Margoliash E et al (1989) A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med 170:1799
Voisine C, Craig EA, Zufall N et al (1999) The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell 97:565
Wadhwa R, Taira K, Kaul SC (2002) An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones 7:309
Whitlow M, Iida K, Marshall P et al (1993) Cells lacking glycan phosphatidylinositol-linked proteins have impaired ability to vesiculate. Blood 81:510
Wiedmer T, Ando B, Sims PJ (1987) Complement C5b-9-stimulated platelet secretion is associated with a Ca2+-initiated activation of cellular protein kinases. J Biol Chem 262:13674
Wiedmer T, Hall SE, Ortel TL et al (1993) Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelets of paroxysmal nocturnal hemoglobinuria. Blood 82:1192
Wiedmer T, Sims PJ (1991) Participation of protein kinases in complement C5b-9-induced shedding of platelet plasma membrane vesicles. Blood 78:2880
Wubbolts R, Leckie RS, Veenhuizen PT et al (2003) Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 278:10963
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilzer, D., Gasser, O., Moskovich, O. et al. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immun 27, 375–387 (2005). https://doi.org/10.1007/s00281-005-0004-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-005-0004-1