Abstract
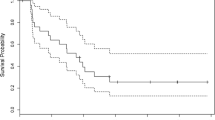
Carmustine (BCNU) has proved to be of value against a variety of primary brain tumors. This agent exhibits a steep dose-response curve in in vitro and animal tumor models and has been proposed for use in high-dose chemotherapy as a single agent or in combination. We conducted a phase II study to assess high-dose BCNU in children with high-grade gliomas. A total of 13 children with high-grade gliomas were treated in a phase II study using high-dose BCNU (800 mg/m2) followed by autologous bone marrow transplantation. Eight patients were newly diagnosed, and five were treated at the time of tumor recurrence. Seven patients had diffuse intrinsic brain-stem gliomas. The response was assessed at 1 month after treatment. Only one objective effect was observed. Five patients had stable disease and seven progressed. The immediate toxicity was mild; however, one patient developed fatal respiratory distress at 50 days after treatment with high-dose BCNU. Dose escalation of BCNU does not seem beneficial in children with high-grade gliomas.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 27 May 1996 / Accepted: 30 August 1996
Rights and permissions
About this article
Cite this article
Bouffet, E., Khelfaoui, F., Philip, I. et al. High-dose carmustine for high-grade gliomas in childhood. Cancer Chemother Pharmacol 39, 376–379 (1997). https://doi.org/10.1007/s002800050586
Issue Date:
DOI: https://doi.org/10.1007/s002800050586