Abstract
Purpose
The aim of the present study was to characterize the pharmacokinetics of irinotecan and its four main metabolites (SN-38, SN-38G, APC and NPC) in metastatic colorectal cancer patients treated with FOLFIRI and FOLFIRINOX regimens and to quantify and explain the inter-individual pharmacokinetic variability in this context.
Methods
A multicenter study including 109 metastatic colorectal cancer patients treated with FOLFIRI or FOLFIRINOX regimen, associated or not with a monoclonal antibody, was conducted. Concentrations of irinotecan and its four main metabolites were measured in 506 blood samples during the first cycle of treatment. Collected data were analyzed using the population approach. First, fixed and random effects models were selected using statistical and graphical methods; second, the impact of covariates on pharmacokinetic parameters was evaluated to explain the inter-individual variability in pharmacokinetic parameters.
Results
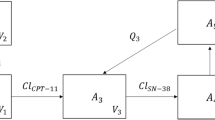
A seven-compartment model best described the pharmacokinetics of irinotecan and its four main metabolites. First-order rates were assigned to distribution, elimination, and metabolism processes, except for the transformation of irinotecan to NPC which was nonlinear. Addition of a direct conversion of NPC into SN-38 significantly improved the model. Co-administration of oxaliplatin significantly modified the distribution of SN-38.
Conclusion
To our knowledge, the present model is the first to allow a simultaneous description of irinotecan pharmacokinetics and of its four main metabolites. Moreover, a direct conversion of NPC into SN-38 had never been described before in a population pharmacokinetic model of irinotecan. The model will be useful to develop pharmacokinetic-pharmacodynamic models relating SN-38 concentrations to efficacy and digestive toxicities.
Clinical trials registration number
ClinicalTrials.gov identifier: NCT00559676.




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The model code that we developed is provided as an electronic supplementary information, so the model can be copy-pasted by any reader and run directly on Monolix® software with the users’ own dataset.
References
de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S (2018) Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet 57:1229–1254
Oyaga-Iriarte E, Insausti A, Sayar O, Aldaz A (2019) Prediction of irinotecan toxicity in metastatic colorectal cancer patients based on machine learning models with pharmacokinetic parameters. J Pharmacol Sci 140:20–25
Mathijssen RH, Van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G et al (2001) Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 7:2182–2194
Catimel G, Chabot GG, Guastalla JP, Dumortier A, Cote C, Engel C et al (1995) Phase I and pharmacokinetic study of irinotecan (CPT-11) administered daily for three consecutive days every three weeks in patients with advanced solid tumors. Ann Oncol 6:133–140
Kehrer DFS, Yamamoto W, Verweij J, de Jonge MJA, de Bruijn P, Sparreboom A (2000) Factors involved in prolongation of the terminal disposition phase of SN-38: clinical and experimental studies. Clin Cancer Res 6:3451–3458
Younis IR, Malone S, Friedman HS, Schaaf LJ, Petros WP (2009) Enterohepatic recirculation model of irinotecan (CPT-11) and metabolite pharmacokinetics in patients with glioma. Cancer Chemother Pharmacol 63:517–524
Loos WJ, Verweij J, Gelderblom HJ, de Jonge MJ, Brouwer E, Dallaire BK et al (1999) Role of erythrocytes and serum proteins in the kinetic profile of total 9-amino-20(S)-camptothecin in humans. Anticancer Drugs 10:705–710
Rivory LP, Haaz MC, Canal P, Lokiec F, Armand JP, Robert J (1997) Pharmacokinetic interrelationships of irinotecan (CPT-11) and its three major plasma metabolites in patients enrolled in phase I/II trials. Clin Cancer Res 3:1261–1266
Substances Irinotécan - VIDAL eVIDAL [Internet]. [cité 14 févr 2020]. Disponible sur: https://evidal-vidal-fr.ezproxy.uca.fr/substance/details/12311/irinotecan.html
Deyme L, Barbolosi D, Gattacceca F (2018) Population pharmacokinetics of FOLFIRINOX: a review of studies and parameters. Cancer Chemother Pharmacol [Internet]. 2018 [cité 21 nov 2018]; Disponible sur: https://doi.org/https://doi.org/10.1007/s00280-018-3722-5
Xie R, Mathijssen RHJ, Sparreboom A, Verweij J, Karlsson MO (2002) Clinical pharmacokinetics of irinotecan and its metabolites in relation with diarrhea. Clin Pharmacol Ther 72:265–275
Poujol S, Pinguet F, Ychou M, Abderrahim AG, Duffour J, Bressolle FMM (2007) A limited sampling strategy to estimate the pharmacokinetic parameters of irinotecan and its active metabolite, SN-38, in patients with metastatic digestive cancer receiving the FOLFIRI regimen. Oncol Rep 18:1613–1321
Mbatchi LC, Robert J, Ychou M, Boyer J-C, Del Rio M, Gassiot M et al (2016) Effect of single nucleotide polymorphisms in the xenobiotic-sensing receptors NR1I2 and NR1I3 on the pharmacokinetics and toxicity of irinotecan in colorectal cancer patients. Clin Pharmacokinet 55:1145–1157
Poujol S, Pinguet F, Malosse F, Astre C, Ychou M, Culine S et al (2003) Sensitive HPLC-fluorescence method for irinotecan and four major metabolites in human plasma and saliva: application to pharmacokinetic studies. Clin Chem 49:1900–1908
Iliadis A (2019) Structural identifiability and sensitivity. J Pharmacokinet Pharmacodyn 46:127–135
Gabrielsson J, Weiner D (2000) Enterohepatic recirculation. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. Swedish Pharmaceutical Press, Stockholm
Petricoul O, Claret L, Barbolosi D, Iliadas A, Puozzo C (2001) Information tools for exploratory data analysis in population pharmacokinetics. J Pharmacokinet Pharmacodyn 28:577–599
Berg AK, Buckner JC, Galanis E, Jaeckle KA, Ames MM, Reid JM (2015) Quantification of the impact of enzyme-inducing antiepileptic drugs on irinotecan pharmacokinetics and SN-38 exposure. J Clin Pharmacol 55:1303–1312
Klein CE, Gupta E, Reid JM, Atherton PJ, Sloan JA, Pitot HC et al (2002) Population pharmacokinetic model for irinotecan and two of its metabolites, SN-38 and SN-38 glucuronide. Clin Pharmacol Ther 72:638–647
Thompson PA, Gupta M, Rosner GL, Yu A, Barrett J, Bomgaars L et al (2008) Pharmacokinetics of irinotecan and its metabolites in pediatric cancer patients: a report from the children’s oncology group. Cancer Chemother Pharmacol 62:1027–1037
Kimura T, Kashiwase S, Makimoto A, Kumagai M, Taga T, Ishida Y et al (2010) Pharmacokinetic and pharmacodynamic investigation of irinotecan hydrochloride in pediatric patients with recurrent or progressive solid tumors. Int J Clin Pharmacol Ther 48:327–334
Mathijssen RHJ, Marsh S, Karlsson MO, Xie R, Baker SD, Verweij J et al (2003) Irinotecan pathway genotype analysis to predict pharmacokinetics. Clin Cancer Res 9:3246–3253
Wasserman E, Cuvier C, Lokiec F, Goldwasser F, Kalla S, Méry-Mignard D et al (1999) Combination of oxaliplatin plus irinotecan in patients with gastrointestinal tumors: results of two independent phase I studies with pharmacokinetics. J Clin Oncol Off J Am Soc Clin Oncol 17:1751–1759
Biswas R, Bugde P, He J, Merien F, Lu J, Liu D-X, et al. Transport-Mediated Oxaliplatin Resistance Associated with Endogenous Overexpression of MRP2 in Caco-2 and PANC-1 Cells. Cancers [Internet]. 2019 [cité 4 juin 2020];11. Disponible sur: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6770320/
Ulrich CM, Robien K, McLeod HL (2003) Cancer pharmacogenetics: polymorphisms, pathways and beyond. Nat Rev Cancer 3:912–920 (Nature Publishing Group)
Asai G, Yamamoto N, Kurata T, Tamura K, Uejima H, Nakagawa K et al (2006) Phase I and pharmacokinetic study of combination chemotherapy using irinotecan and paclitaxel in patients with lung cancer. J Thorac Oncol 1:226–230
Gupta E, Lestingi TM, Mick R, Ramirez J, Vokes EE, Ratain MJ (1994) Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res Am Assoc Cancer Res 54:3723–5
Ecker GF, Chiba P (2009) Transporters as Drug Carriers: Structure, Function, Substrates. John Wiley & Sons
Acknowledgements
The authors gratefully acknowledge Professor A. Iliadis for the assistance and the fruitful discussions regarding the model development. They would also like to thank the ligue Contre le Cancer French association who generously provided a PhD grant to Laure Deyme.
Funding
This work was supported by a Grant from Cancéropôle Grand Sud-Ouest (France).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Laure Deyme, Dominique Barbolosi, Litaty Céphanoée Mbatchi, Nicole Tubiana-Mathieu, Marc Ychou, Alexandre Evrard, and Florence Gattacceca declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
280_2021_4255_MOESM1_ESM.pdf
Supplementary file1 The model code that we developed is provided as an electronic supplementary information, so the model can be copy-pasted by any reader and run directly on Monolix® software with the users’ own dataset. Additional GOF plots for CPT-11 and metabolites, including normalized prediction distribution errors, are provided in Supplementary File 2 (PDF 16 KB)
Rights and permissions
About this article
Cite this article
Deyme, L., Barbolosi, D., Mbatchi, L.C. et al. Population pharmacokinetic model of irinotecan and its four main metabolites in patients treated with FOLFIRI or FOLFIRINOX regimen. Cancer Chemother Pharmacol 88, 247–258 (2021). https://doi.org/10.1007/s00280-021-04255-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04255-9