Abstract
Purpose
Time is a critical factor in drug action. The duration of inhibition of the target or residence time of the drug molecule on the target often guides drug scheduling.
Methods
The effects of time on the concentration-dependent cytotoxicity of approved and investigational agents [300 compounds] were examined in the NCI60 cell line panel in 2D at 2, 3, 7 and in 3D 11 days.
Results
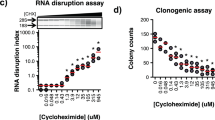
There was a moderate positive linear relationship between data from the 2-day NCI60 screen and the 3-, 7- and 11-day and a strong positive linear relationship between 3-, 7- and 11-day luminescence screen IC50s by Pearson correlation analysis. Cell growth inhibition by agents selective for a specific cell cycle phase plateaued when susceptible cells were growth inhibited or killed. As time increased the depth of cell growth inhibition increased without change in the IC50. DNA interactive agents had decreasing IC50s with increasing exposure time. Epigenetic agents required longer exposure times; several were only cytotoxic after 11 days’ exposure. For HDAC inhibitors, time had little or no effect on concentration response. There were potency differences amongst the three BET bromodomain inhibitors tested, and an exposure duration effect. The PARP inhibitors, rucaparib, niraparib, and veliparib reached IC50s < 10 μM in some cell lines after 11 days.
Conclusions
The results suggest that variations in compound exposure time may reflect either mechanism of action or compound chemical half-life. The activity of slow-acting compounds may optimally be assessed in spheroid models that can be monitored over prolonged incubation times.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Eastman A (2017) Improving anticancer drug development begins with cell culture: misinformation perpetrated by the misuse of cytotoxicity assays. Oncotarget 8:8854–8866
Perego P, Hempel G, Linder S, Bradshaw TD, Larsen AK, Peters GJ, Phillips RM, on behalf of the EORTC PAMM Group (2018) Cellular pharmacology studies of anticancer agents: recommendations from the EORTC-PAMM group. Cancer Chemother Pharmacol 81:427–41
Liston DR, Davis M (2017) Clinically relevant concentrations of anticancer drugs: a guide for nonclinical studies. Clin Cancer Res 23:3489–3498
Henderson ES, Adamson RH, Denham C, Oliverio VT (1965) The metabolic fate of tritiated methotrexate I. absorption, excretion and distribution in mice, rats, dogs and monkeys. Cancer Res 25:1008–1017
Ludwig R, Alberts DS (1984) Chemical and biological stability of anticancer drugs used in a human tumor clonogenic assay. Cancer Chemother Pharmacol 12:142–145
Beijnen JH, Van der Nat JM, Labadie RP, Underberg WJ (1986) Decomposition of mitomycin and anthracycline cytostatics in cell culture media. Anticancer Res 6:39–43
Niell HD, Webster KC, Smith EE (1985) Anticancer drug activity in platin in human bladder tumor cell lines. Cancer 56:1039–1044
Schuldes H, Bade S, Knobloch J, Jonas D (1997) Loss of in vitro cytotoxicity of cisplatin after storage as stock solution in cell culture medium at various temperatures. Cancer 79:1723–1728
Karahoca M, Momparler RL (2013) Pharmacokinetic and pharmacodynamic analysis of 5-aza-2’-deoxycytidine (decitabine) in the design of its dose-schedule for cancer therapy. Clin Epigen 5:3–19
Du L, Musson DG, Wang AQ (2006) Stability studies of vorinostat and its two metabolites in human plasma, serum and urine. J Pharmaceut Biomed Anal 42:556–564
Plumb JA, Finn PW, Williams RJ, Bandara MJ, La Thangue NB, Brown R (2003) Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Therap 2:721–728
Wang H, Yu N, Chen D, Lee KCL, Lye PL, Chang JWW, Deng W, Ng MCY, Lu T et al (2011) Discovery of (2E)-3-{2-butyl-1-[2-(diethylamino)ethyl]-1H-benzimidazol-5-yl}-N-hydroxyacrylamide (SB939), an orally active histone deacetylase inhibitor with a superior preclinical profile. J Med Chem 54:4694–4720
Konsoula R, Jung M (2008) In vitro plasma stability, permeability and solubility of mercaptoacetamide histone deacetylase inhibitors. Int J Pharm 361:19–25
Holbeck SL, Camalier R, Crowell JA, Govinharajulu JP, Hollingshead M, Anderson LW, Polley E, Rubenstein L, Srivastava A, Wilsker D, Collins JM, Doroshow JH (2017) The national cancer institute ALMANAC: a comprehensive screening resource for the detection of anticancer drug pairs with enhanced therapeutic activity. Cancer Res 77:3564–3576
Lorenzi PL, Reinhold WC, Varma S, Hutchinson AA, Pommier Y, Chanock SJ, Weinstein J (2009) DNA fingerprinting of the NCI-60 cell line panel. Mol Cancer Ther 8:713–724
Selby M, Delosh R, Laudeman J, Ogle C, Reinhart R, Silvers T, Lawrence S, Kinders R, Parchment R, Teicher BA, Evans DM (2017) 3D models of the NCI60 cell lines for screening oncology compounds. SLAS Discovery 22:473–483
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS ONE 10(12):e0146021
Shoemaker RH (2006) The NCI60 human tumor cell line anticancer drug screen. Nat Rev Cancer 6:813–823
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell P, Mayo J, Boyd M (1991) Feasibility of a High-flux anticancer drug screen using a diverse panel of cultured tumor cell lines. J Natl Cancer Inst 83:757–766
Weinstein JN, Myers TG, O’Connor PM, Friend SH, Fornace AJ, Kohn KW, Fojo T, Bates SE, Rubinstein LV, van Osdol WW, Monks AP et al (1997) An informative-intensive approach to the molecular pharmacology of cancer. Science 275:343–349
Bertino JR (2009) Cancer research: from folate antagonism to molecular targets. Best Prac Res Clin Hematol 22:577–582
Li H, Li W, Liu S, Zong S, Wang W, Ren J, Li Q, Hou F, Shi Q (2016) DNMT1, DNMT3A and DNMT3B polymorphisms associated with gastric cancer risk: a systemic review and meta-analysis. EBioMed 13:125–131
Uysal F, Akkoyunlu G, Ozturk S (2015) Dynamic expression of DNA methyltransferase (DNMTs) in oocytes and early embryos. Biochimie 116:103–113
Fahy J, Jeltsch A, Arimondo PB (2012) DNA methyltransferase inhibitors in cancer: a chemical and therapeutic patent overview and selected clinical studies. Exp Opin Ther Patents 22:1427–1442
Hollenbach PW, Nguyen AN, Brady H, Williams M, Ning Y, Richard N, Krushel L, Aukerman SL, Heise C, MacBeth KJ (2010) A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS ONE 5:e9001
Yoo J, Choi S, Medina-Franco JL (2013) Molecular modeling studies of the novel inhibitors of DNA methyltransferases SGI-1027 and CBC12: implications for the mechanism of inhibition of DNMTs. PLoS ONE 8:e62152
Peters GJ, Smid K, Vecchi L, Kathmann I, Sarksjan D, Honeywell RJ, Losekoot N, Ohne O, Orbach A, Blaugrund E, Jeong LS, Lee YB, Ahn CH, Kim DJ (2013) Metabolism, mechanism of action and sensitivity profile of fluorocyclopentenylcytosine (RX-3117; TV-1360). Invest New Drugs 31:1444–1457
Eckschlager T, Plch J, Stiborova M, Hrabeta J (2017) Histone deacetylase inhibitors as anticancer drugs. Int J Molec Sci 18:1414–1439
Shen L, Orillion A, Pili R (2016) Histone deacetylase inhibitors as immunomodulators in cancer therapeutics. Epigenomics 8:415–428
Kim KH, Roberts CWM (2016) Targeting EZH2 in cancer. Nat Med 22:128–134
Italiano A (2016) Role of the EZH2 histone methyltransferase as a therapeutic target in cancer. Pharm Therap 165:26–31
Lu C, Figueroa JA, Liu Z, Konala V, Aulakhy A, Verma R, Cobos E, Chiriva-Internati M, Gao W (2015) Nuclear export as a novel therapeutic target: the CRM1 connection. Curr Drug Targets 15:575–592
El-Tanani M, Dakir E, Raynor B, Morgan R (2016) Mechanisms of nuclear export in cancer and resistance to chemotherapy. Cancers 8:35–46
Garg M, Kanojia D, Mayakonda A, Said JW, Doan NB, Chien W, Ganesan TS, Chuang LSH et al (2017) Molecular mechanism and therapeutic implications of selinexor (KPT-330) in liposarcoma. Oncotarget 8:7521–7532
Magina KN, Pregartner G, Zebisch A, Wolfler A, Neumeister P, Greinix HT, Berghold A, Sill H (2017) Cytarabine dose in the consolidation of AML: a systematic review and meta-analysis. Blood 130:946–948
Zhenchuk A, Lotfi K, Juliusson G, Albertioni F (2009) Mechanisms of anti-cancer action and pharmacology of clofarabine. Biochem Pharmacol 78:1351–1359
Jung M, Gelato KA, Fernandez-Montalvan A, Siegel S, Haendler B (2015) Targeting BET bromodomains for cancer treatment. Epigenomics 7:487–501
Andrieu G, Belkina AC, Denis GV (2016) Clinical trials for BET inhibitors run ahead of the science. Drug Discov Today 19:45–50
Sahai V, Redig AJ, Collier KA, Eckerdt FD, Munshi HG (2016) Targeting BET bromodomain proteins in solid tumors. Oncotarget 7:53997–54009
Xu Y, Vakoc CR (2017) Targeting cancer cells with BET bromodomain inhibitors. Cold Spring Harbor Perspect Med 7:a026674
Brown JS, O’Carrigan B, Jackson SP, Yap TA (2017) Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov 7:20–37
Lord CJ, Ashworth A (2017) PARP inhibitors: synthetic lethality in the clinic. Science 355:1152–1158
Probst BL, Liu L, Ramesh V, Li L, Sun H, Minna JD, Wang L (2010) Smac mimetics increase cancer cell response to chemotherapeutics in a TNF-α-dependent manner. Cell Death Differ 17:1645–1654
Gonnissen A, Isebaert S, Haustermans K (2015) Targeting the hedgehog signaling pathway in cancer: beyond smoothened. Oncotarget 6:13899–13913
Rimkus TK, Carpenter RI, Qasem S, Chan M, Lo HW (2016) Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers 8:22–45
Derakhshan A, Chen Z, Van Waes C (2016) Therapeutic small molecules target inhibitor of apoptosis proteins in cancers with deregulation of extrinsic and intrinsic cell death pathways. Clin Cancer Res 23:1379–1387
Peery RC, Liu JY, Zhang JT (2017) Targeting survivin for therapeutic discovery: past, present, and future promises. Drug Discov Today 22:1466–1477
Altieri DC (2010) Survivin and IAP proteins in cell-death mechanisms. Biochem J 430:199–205
Zhang H (2016) Three generations of epidermal growth factor receptor tyrosine kinase inhibitors developed to revolutionize the therapy of lung cancer. Drug Design Dev Therapy 10:3867–3872
Santarpia M, Ligori A, Karachaliou N, Gonzalez-Cao M, Daffina MG, D’Aveni A, Marabello G, Altavilla G, Rosell R (2017) Osimertinib in the treatment of non-small cell lung cancer: design, development and place in therapy. Lung Cancer Targets Therapy 8:109–125
Gaumann AKA, Kiefer F, Alfer J, Lang SA, Geissler EK, Breier G (2016) Receptor tyrosine kinase inhibitors: are they real tumor killers? Int J Cancer 138(540–54):52
Wengner AM, Siemeister G, Koppitz M, Schulze V, Kosemund D, Klar U, Stoeckigt D, Neuhaus R, Lienau P et al (2016) Novel MPS1 kinase inhibitors with potent antitumor activity. Molec Cancer Therap 15:583–592
Ma WW (2011) Development of focal adhesion kinase inhibitors in cancer therapy. Anti-cancer Agents Med Chem 11:638–642
Ray GR, Hahn GM, Bagshaw MA, Kurkjian S (1973) Cell survival and repair of plateau phase cultures after chemotherapy: relevance to tumor therapy and to the in vitro screening of new agents. Cancer Chemother Rep 57:473–475
Keyes K, Cox K, Treadway P, Mann L, Shih C, Faul MM, Teicher BA (2002) An in vitro tumor model: analysis of angiogenic factor expression after chemotherapy. Cancer Res 62:5597–5602
Bale SS, Moore L, Yarmush M, Jindal R (2016) Emerging in vitro liver technologies for drug metabolism and inter-organ interactions. Tissue Engineer B 22:383–394
Pelkonen O, Turpeinen M, Hakkola J, Abass K, Pasanen M, Raunio H, Vahakangas K (2013) Preservation, induction or incorporation of metabolism into the in vitro cellular system—views to current opportunities and limitations. Toxicol In Vitro 27:1578–1583
Barrero ML (2017) Epigenetic strategies to boost cancer immunotherapies. Int J Mol Sci 18:1108–1127
Halsall JA, Turner BM (2016) Histone deacetylase inhibitors for cancer therapy. An evolutionarily ancient resistance response may explain their limited success. Bioessays 38:1102–1110
Zhou MM, Dhalliun C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM (1999) Structure and ligand of a histone acetyltransferase bromodomain. Nature 399:491–496
McGlynn O, Lloyd B (2002) Recombinational repair and restart of damaged replication forks. Nat Rev 3:859–870
Fleuren EDG, Zhang L, Wu J, Daly RJ (2016) The kinome ‘at large’ in cancer. Nat Rev Cancer 16:83–98
Rotow J, Bivona TG (2017) Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 17:637–658
Heerboth S, Lapinska K, Snyder N, Leary M, Rollinson S, Sarkar S (2014) Use of epigenetic drugs in disease: an overview. Genet Epigenet 6:9–19
Acknowledgements
This project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors state that they have no conflicts of interest to report. The study does not involve human subjects or use of animals in research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2019_3863_MOESM2_ESM.pptx
Supplemental Figure 1. Median concentrations (micromolar, blue) and mean log IC50 concentrations (micromolar, red) for the NCI60 cell line panel after compound exposure for 2 days, 3 days, 7 days or 11 days for methotrexate, raltitrexed, doxorubicin, topotecan, belinostat, panobinostat, romidepsin and selinexor. Supplemental Figure 2A. Mean log IC50 concentrations for the NCI60 cell line panel after compound exposure for 2 days, 3 days, 7 days or 11 days for the EGFR inhibitor erlotinib (blue), the FAK inhibitor TAE-226, the MPS-1 inhibitor BAY-1217389 and for the Akt inhibitor MK-2206. 2B. Mean log IC50 concentrations for the NCI60 cell line panel after compound exposure for 2 days, 3 days, 7 days or 11 days for the BET bromodomain inhibitors MK8628, BET-BAY-002 and GSK-525762. 2C. Mean log IC50 concentrations for the NCI60 cell line panel after compound exposure for 2 days, 3 days, 7 days or 11 days for the IAP inhibitors birinapant and LCL-161. Supplemental Figure 3A. Concentration response curves in three representative NCI60 panel cell lines after compound exposure for 2 days (blue), 3 days (red), 7 days (yellow) or 11 days (green) for the cell surface transmembrane receptor tyrosine kinase inhibitors sorafenib, erlotinib, crizotinib and nintedanib. B. Concentration response curves in three representative NCI60 panel cell lines after compound exposure for 2 days (blue), 3 days (red), 7 days (yellow) or 11 days (green) for selected intracellular kinases: FAK inhibitors TAE-226, MPS-1 inhibitor BAY-1217389, and Akt inhibitor MK-2206 (PPTX 2132 kb)
Rights and permissions
About this article
Cite this article
Evans, D.M., Fang, J., Silvers, T. et al. Exposure time versus cytotoxicity for anticancer agents. Cancer Chemother Pharmacol 84, 359–371 (2019). https://doi.org/10.1007/s00280-019-03863-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03863-w