Abstract
Purpose
To validate the presence of serine–threonine kinase receptor-associated Protein (STRAP) in osteosarcoma tissue and to investigate the oncological role of STRAP in osteosarcoma.
Methods
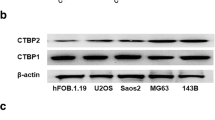
Expression of STRAP protein in osteosarcoma tissue compared to soft callus (hyperactive bone healing tissue) and in multiple cell lines was examined using western blot analysis. Effects of STRAP silencing on cell proliferation, invasion, migration and re-implantability in chick chorioallantoic membrane (CAM) were observed in osteosarcoma cell lines (MNNG-HOS, 143B, and U2OS).
Results
The result demonstrated that STRAP was highly up-regulated in osteosarcoma tissues compared with the normal physiological bone healing tissue (soft callus). Expression level of STRAP was markedly high in osteosarcoma cell lines with aggressive phenotype. Upon STRAP silencing, invasion and migration, but not proliferative activity, were selectively modulated in high-expression-STRAP cell lines. In addition, STRAP silencing reduced the success rate of tumor implantation and growth of MNNG-HOS cells in CAM model.
Conclusions
Serine–threonine kinase receptor-associated protein is up-regulated during osteosarcoma progression. The presence of STRAP enhances osteosarcoma cell invasion, migration and re-implantation ability, factors which play a critical role in metastasis. Serine–threonine kinase receptor-associated protein and its related pathway are worthy for further exploration as a novel target for anti-metastasis agents.





Similar content being viewed by others
References
Tang N, Song WX, Luo J, Haydon RC, He TC (2008) Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res 466(9):2114–2130. https://doi.org/10.1007/s11999-008-0335-z
Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20(3):776–790
Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR (2012) A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012:704872. https://doi.org/10.1155/2012/704872
Broadhead ML, Clark JC, Myers DE, Dass CR, Choong PF (2011) The molecular pathogenesis of osteosarcoma: a review. Sarcoma 2011:959248. https://doi.org/10.1155/2011/959248
Carano RA, Filvaroff EH (2003) Angiogenesis and bone repair. Drug Discov Today 8(21):980–989
Datta PK, Moses HL (2000) STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling. Mol Cell Biol 20(9):3157–3167
Chen G, Deng C, Li YP (2012) TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8(2):272–288. https://doi.org/10.7150/ijbs.2929
Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K (2004) Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J 23(3):552–563. https://doi.org/10.1038/sj.emboj.7600067
Vukmirovic M, Manojlovic Z, Stefanovic B (2013) Serine-threonine kinase receptor-associated protein (STRAP) regulates translation of type I collagen mRNAs. Mol Cell Biol 33(19):3893–3906. https://doi.org/10.1128/MCB.00195-13
Halder SK, Anumanthan G, Maddula R, Mann J, Chytil A, Gonzalez AL, Washington MK, Moses HL, Beauchamp RD, Datta PK (2006) Oncogenic function of a novel WD-domain protein, STRAP, in human carcinogenesis. Cancer Res 66(12):6156–6166. https://doi.org/10.1158/0008-5472.CAN-05-3261
Franchi A, Arganini L, Baroni G, Calzolari A, Capanna R, Campanacci D, Caldora P, Masi L, Brandi ML, Zampi G (1998) Expression of transforming growth factor beta isoforms in osteosarcoma variants: association of TGF beta 1 with high-grade osteosarcomas. J Pathol 185 (3):284–289. https://doi.org/10.1002/(SICI)1096-9896(199807)185:3%3C284::AID-PATH94%3E3.0.CO;2-Z
Weil D, Garçon L, Harper M, Duménil D, Dautry F, Kress M (2002) Targeting the kinesin Eg5 to monitor siRNA transfection in mammalian cells. Biotechniques 33(6):1244–1248
Pruksakorn D, Teeyakasem P, Klangjorhor J, Chaiyawat P, Settakorn J, Diskul-Na-Ayudthaya P, Chokchaichamnankit D, Pothacharoen P, Srisomsap C (2016) Overexpression of KH-type splicing regulatory protein regulates proliferation, migration, and implantation ability of osteosarcoma. Int J Oncol 49(3):903–912. https://doi.org/10.3892/ijo.2016.3601
Balke M, Neumann A, Kersting C, Agelopoulos K, Gebert C, Gosheger G, Buerger H, Hagedorn M (2010) Morphologic characterization of osteosarcoma growth on the chick chorioallantoic membrane. BMC Res Notes 3:58. https://doi.org/10.1186/1756-0500-3-58
Ribatti D, Nico B, Morbidelli L, Donnini S, Ziche M, Vacca A, Roncali L, Presta M (2001) Cell-mediated delivery of fibroblast growth factor-2 and vascular endothelial growth factor onto the chick chorioallantoic membrane: endothelial fenestration and angiogenesis. J Vasc Res 38(4):389–397. doi:51070
Chaiyawat P, Pruksakorn D, Phanphaisarn A, Teeyakasem P, Klangjorhor J, Settakorn J (2017) Expression patterns of class I histone deacetylases in osteosarcoma: a novel prognostic marker with potential therapeutic implications. Mod Pathol. https://doi.org/10.1038/modpathol.2017.125
Reiner JE, Datta PK (2011) TGF-beta-dependent and -independent roles of STRAP in cancer. Front Biosci (Landmark Ed) 16:105–115
Datta PK, Chytil A, Gorska AE, Moses HL (1998) Identification of STRAP, a novel WD domain protein in transforming growth factor-beta signaling. J Biol Chem 273(52):34671–34674
Xu S, Yang S, Sun G, Huang W, Zhang Y (2014) Transforming growth factor-beta polymorphisms and serum level in the development of osteosarcoma. DNA Cell Biol 33(11):802–806. https://doi.org/10.1089/dna.2014.2527
Lamora A, Talbot J, Bougras G, Amiaud J, Leduc M, Chesneau J, Taurelle J, Stresing V, Le Deley MC, Heymann MF, Heymann D, Redini F, Verrecchia F (2014) Overexpression of smad7 blocks primary tumor growth and lung metastasis development in osteosarcoma. Clin Cancer Res 20(19):5097–5112. https://doi.org/10.1158/1078-0432.CCR-13-3191
Lamora A, Mullard M, Amiaud J, Brion R, Heymann D, Redini F, Verrecchia F (2015) Anticancer activity of halofuginone in a preclinical model of osteosarcoma: inhibition of tumor growth and lung metastases. Oncotarget 6(16):14413–14427. https://doi.org/10.18632/oncotarget.3891
Lauvrak SU, Munthe E, Kresse SH, Stratford EW, Namlos HM, Meza-Zepeda LA, Myklebost O (2013) Functional characterisation of osteosarcoma cell lines and identification of mRNAs and miRNAs associated with aggressive cancer phenotypes. Br J Cancer 109(8):2228–2236. https://doi.org/10.1038/bjc.2013.549
Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S (2004) Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res 24(6):3743–3748
Tejeda-Munoz N, Robles-Flores M (2015) Glycogen synthase kinase 3 in Wnt signaling pathway and cancer. IUBMB Life 67(12):914–922. https://doi.org/10.1002/iub.1454
Yuan G, Zhang B, Yang S, Jin L, Datta A, Bae S, Chen X, Datta PK (2016) Novel role of STRAP in progression and metastasis of colorectal cancer through Wnt/beta-catenin signaling. Oncotarget 7(13):16023–16037. https://doi.org/10.18632/oncotarget.7532
Yilmaz M, Christofori G (2010) Mechanisms of motility in metastasizing cells. Mol Cancer Res 8(5):629–642. https://doi.org/10.1158/1541-7786.MCR-10-0139
Jin L, Datta PK (2014) Oncogenic STRAP functions as a novel negative regulator of E-cadherin and p21(Cip1) by modulating the transcription factor Sp1. Cell Cycle 13(24):3909–3920. https://doi.org/10.4161/15384101.2014.973310
Demir R, Dimmler A, Naschberger E, Demir I, Papadopoulos T, Melling N, Sturzl M, Hohenberger W (2009) Malignant progression of invasive tumour cells seen in hypoxia present an accumulation of beta-catenin in the nucleus at the tumour front. Exp Mol Pathol 87(2):109–116. https://doi.org/10.1016/j.yexmp.2009.05.004
Krakhmal NV, Zavyalova MV, Denisov EV, Vtorushin SV, Perelmuter VM (2015) Cancer invasion: patterns and mechanisms. Acta Naturae 7(2):17–28
Lokman NA, Elder AS, Ricciardelli C, Oehler MK (2012) Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int J Mol Sci 13(8):9959–9970. https://doi.org/10.3390/ijms13089959
Ribatti D (2017) The chick embryo chorioallantoic membrane (CAM) assay. Reprod Toxicol 70:97–101. https://doi.org/10.1016/j.reprotox.2016.11.004
Acknowledgements
This study was supported by Thailand Research Fund (TRF): MRG5980213; Orthopedic Laboratory and Research Network (OLARN), Faculty of Medicine, Chiang Mai University; National Research University (NRU) fund. The authors would also like to express their sincere thanks Dr. G. Lamar Robert and Assoc. Prof. Dr. Chongchit Sripun Robert for their manuscript proofreading.
Funding
This study was funded by Thailand Research Fund (TRF): MRG5980213
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed (EC No. 23/2559 for Chick Chorioallantoic Membrane (CAM) assay for animal study, Chiang Mai University). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (OLARN Tissue Bank, Faculty of Medicine, CMU, EC-No: ID2717, Study code: ORT-2557-02717), and research protocols in this study were approved by the Ethical committee of the Faculty of Medicine, Chiang Mai University (CMU), Chiang Mai Thailand: EC No. ORT-11-09-16A-14 (ID#757).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pruksakorn, D., Klangjorhor, J., Lirdprapamongkol, K. et al. Oncogenic roles of serine–threonine kinase receptor-associated protein (STRAP) in osteosarcoma. Cancer Chemother Pharmacol 82, 1039–1047 (2018). https://doi.org/10.1007/s00280-018-3696-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3696-3