Abstract
Background
The aim of this single-arm phase II clinical trial was to evaluate whether the alternate-day administration of S-1 plus irinotecan would reduce the incidence of severe diarrhea in comparison to consecutive-day S-1 administration (standard IRIS regimen) in second-line treatment for patients with metastatic colorectal cancer.
Methods
Patients with metastatic colorectal cancer after failure with first-line treatment of oxaliplatin and fluoropyrimidine were enrolled. Irinotecan (150 mg/m2) and bevacizumab (5 mg/kg) were given intravenously on day 1. Oral S-1 was administered on alternate days at a dose of 40–60 mg twice a day. Cycles were repeated every 2 weeks. The primary endpoint was the incidence of grade ≥ 3 diarrhea. Our hypothesis set 21% as a threshold incidence and 10% as an expected incidence from previous studies with one-sided alpha 0.05. The secondary endpoints included the relative dose intensity, progression-free survival, overall survival and other adverse events.
Results
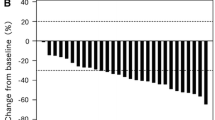
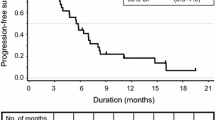
A total of 51 patients were enrolled. The incidence of grade ≥ 3 diarrhea was 15.7% (8/51). Other common grade ≥ 3 adverse events were neutropenia, anemia, thrombocytopenia and fatigue were 13.7% (7/51), 5.9% (3/51), 2.0% (1/51) and 5.9% (3/51), respectively. The relative dose intensities of irinotecan, bevacizumab, and S-1 were 80.0, 86.8, and 77.7%, respectively. The median progression-free survival and overall survival were 8.4 months (5.8–9.8) and 17.1 months (11.8–22.3).
Conclusions
The alternate-day S-1 administration does not have significant effectiveness to reduce diarrhea in patients who received second-line treatment for metastatic colorectal cancer.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22(7):1209–1214. https://doi.org/10.1200/jco.2004.11.037
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947
Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Levi F (2000) Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 18(1):136–147. https://doi.org/10.1200/jco.2000.18.1.136
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355(9209):1041–1047
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22(2):229–237. https://doi.org/10.1200/jco.2004.05.113
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360(14):1408–1417. https://doi.org/10.1056/NEJMoa0805019
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. https://doi.org/10.1056/NEJMoa032691
Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, Chirivella I, Perez-Staub N, Louvet C, Andre T, Tabah-Fisch I, de Gramont A (2006) OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol 24(3):394–400. https://doi.org/10.1200/JCO.2005.03.0106
Mabro M, Artru P, Andre T, Flesch M, Maindrault-Goebel F, Landi B, Lledo G, Plantade A, Louvet C, de Gramont A (2006) A phase II study of FOLFIRI-3 (double infusion of irinotecan combined with LV5FU) after FOLFOX in advanced colorectal cancer patients. Br J Cancer 94(9):1287–1292. https://doi.org/10.1038/sj.bjc.6603095
Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, Yamaguchi K, Takiuchi H, Esaki T, Tokunaga S, Kuwano H, Komatsu Y, Watanabe M, Hyodo I, Morita S, Sugihara K (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11(9):853–860. https://doi.org/10.1016/s1470-2045(10)70181-9
Arai W, Hosoya Y, Hyodo M, Yokoyama T, Hirashima Y, Yasuda Y, Nagai H, Shirasaka T (2004) Alternate-day oral therapy with TS-1 for advanced gastric cancer. Int J Clin Oncol 9(3):143–148. https://doi.org/10.1007/s10147-004-0381-9
Sakuma K, Hosoya Y, Arai W, Haruta H, Ui T, Kurashina K, Saito S, Hirashima Y, Yokoyama T, Zuiki T, Hyodo M, Nagai H, Yasuda Y, Shirasaka T (2010) Alternate-day treatment with S-1 in patients with gastric cancer: a retrospective study of strategies for reducing toxicity. Int J Clin Oncol 15(2):166–171. https://doi.org/10.1007/s10147-010-0036-y
Komura T, Miura K, Shirasaka T, Ohnuma S, Shimada M, Kajiwara T, Fujishima F, Philchenkov A, Nakagawa K, Kudoh K, Haneda S, Toshima M, Kohyama A, Musha H, Naitoh T, Shibata C, Unno M (2015) Usefulness of alternate-day administration of S-1 and leucovorin in a xenograft mouse model of colorectal cancer: a shorter drug-free interval leads to more efficient antitumor effects. Int J Clin Oncol 20(1):117–125. https://doi.org/10.1007/s10147-014-0699-x
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford: 1990) 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Agresti A, Coull B (1998) Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52(2):119–126
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Saltz L (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26(12):2006–2012. https://doi.org/10.1200/JCO.2007.14.9898
Doi T, Boku N, Kato K, Komatsu Y, Yamaguchi K, Muro K, Hamamoto Y, Sato A, Koizumi W, Mizunuma N, Takiuchi H (2010) Phase I/II study of capecitabine plus oxaliplatin (XELOX) plus bevacizumab as first-line therapy in Japanese patients with metastatic colorectal cancer. Jpn J Clin Oncol 40(10):913–920. https://doi.org/10.1093/jjco/hyq069
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Rittweger K, Gilberg F, Saltz L (2011) XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer 105(1):58–64. https://doi.org/10.1038/bjc.2011.201
Matsuda C, Honda M, Tanaka C, Fukunaga M, Ishibashi K, Munemoto Y, Hata T, Bando H, Oshiro M, Kobayashi M, Tokunaga Y, Fujii A, Nagata N, Oba K, Mishima H (2015) Multicenter randomized phase II clinical trial of oxaliplatin reintroduction as a third- or later-line therapy for metastatic colorectal cancer-biweekly versus standard triweekly XELOX (the ORION Study). Int J Clin Oncol. https://doi.org/10.1007/s10147-015-0911-7
Yamada Y, Tahara M, Miya T, Satoh T, Shirao K, Shimada Y, Ohtsu A, Sasaki Y, Tanigawara Y (2008) Phase I/II study of oxaliplatin with oral S-1 as first-line therapy for patients with metastatic colorectal cancer. Br J Cancer 98(6):1034–1038. https://doi.org/10.1038/sj.bjc.6604271
Sato Y, Ohnuma H, Hirakawa M, Takahashi M, Osuga T, Okagawa Y, Murase K, Takada K, Kawano Y, Iyama S, Hayashi T, Sato T, Miyanishi K, Takimoto R, Kobune M, Okita K, Mizuguchi T, Furuhata T, Hirata K, Kato J (2015) A dose-escalation study of oxaliplatin/capecitabine/irinotecan (XELOXIRI) and bevacizumab as a first-line therapy for patients with metastatic colorectal cancer. Cancer Chemother Pharmacol 75(3):587–594. https://doi.org/10.1007/s00280-014-2672-9
Hamamoto Y, Yamaguchi T, Nishina T, Yamazaki K, Ura T, Nakajima T, Goto A, Shimada K, Nakayama N, Sakamoto J, Morita S, Yamada Y (2014) A phase I/II study of XELIRI plus bevacizumab as second-line chemotherapy for Japanese patients with metastatic colorectal cancer (BIX study). Oncologist 19(11):1131–1132. https://doi.org/10.1634/theoncologist.2014-0159
Komatsu Y, Yuki S, Sogabe S, Fukushima H, Iwanaga I, Kudo M, Tateyama M, Meguro T, Uebayashi M, Saga A, Sakata Y, Asaka M (2011) Phase II study of combined treatment with irinotecan and S-1 (IRIS) in patients with inoperable or recurrent advanced colorectal cancer (HGCSG0302). Oncology 80(1–2):70–75. https://doi.org/10.1159/000328739
Yamada Y, Yamaguchi T, Matsumoto H, Ichikawa Y, Goto A, Kato K, Hamaguchi T, Shimada Y (2012) Phase II study of oral S-1 with irinotecan and bevacizumab (SIRB) as first-line therapy for patients with metastatic colorectal cancer. Investig New Drugs 30(4):1690–1696. https://doi.org/10.1007/s10637-011-9743-0
Kajiwara T, Miura K, Ohnuma S, Shimada M, Komura T, Toshima M, Kohyama A, Kudoh K, Haneda S, Musha H, Naitoh T, Shirasaka T, Unno M (2015) Gastrointestinal toxicities of 5-fluorouracil increase the proportion of regulatory T cells in intestinal tract: advantages of alternate-day S-1 administration. Int J Clin Oncol 20(5):913–921. https://doi.org/10.1007/s10147-015-0791-x
Hoff PM, Saragiotto DF, Barrios CH, del Giglio A, Coutinho AK, Andrade AC, Dutra C, Forones NM, Correa M, Portella Mdo S, Passos VQ, Chinen RN, van Eyll B (2014) Randomized phase III trial exploring the use of long-acting release octreotide in the prevention of chemotherapy-induced diarrhea in patients with colorectal cancer: the LARCID trial. J Clin Oncol 32(10):1006–1011. https://doi.org/10.1200/jco.2013.50.8077
Kee BK, Morris JS, Slack RS, Crocenzi T, Wong L, Esparaz B, Overman M, Glover K, Jones D, Wen S, Fisch MJ (2015) A phase II, randomized, double blind trial of calcium aluminosilicate clay versus placebo for the prevention of diarrhea in patients with metastatic colorectal cancer treated with irinotecan. Support Care Cancer 23(3):661–670. https://doi.org/10.1007/s00520-014-2402-1
Karthaus M, Ballo H, Abenhardt W, Steinmetz T, Geer T, Schimke J, Braumann D, Behrens R, Behringer D, Kindler M, Messmann H, Boeck HP, Greinwald R, Kleeberg U (2005) Prospective, double-blind, placebo-controlled, multicenter, randomized phase III study with orally administered budesonide for prevention of irinotecan (CPT-11)-induced diarrhea in patients with advanced colorectal cancer. Oncology 68(4–6):326–332. https://doi.org/10.1159/000086971
Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B, Beniak J, Medvecova L, Lagin A, Svetlovska D, Spanik S, Zajac V, Mardiak J, Drgona L (2015) Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med 23(3):356–362. https://doi.org/10.1016/j.ctim.2015.03.008
Osterlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P, Kouri M, Elomaa I, Joensuu H (2007) Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer 97(8):1028–1034. https://doi.org/10.1038/sj.bjc.6603990
Funding
This study fund was provided to ECRIN by Taiho Pharmaceutical Co., Ltd., under the study contract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JS has received honoraria from Tsumura, Chugai Pharmaceutical, and consulting fee from Takeda Pharmaceutical. HM has received consulting fee from Takeda Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical and Taiho Pharmaceutical. Other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Matsuda, C., Honda, M., Tanaka, C. et al. A phase II study of bevacizumab and irinotecan plus alternate-day S-1 as a second-line therapy in patients with metastatic colorectal cancer: the AIRS study. Cancer Chemother Pharmacol 81, 1035–1041 (2018). https://doi.org/10.1007/s00280-018-3568-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3568-x