Abstract
Purpose
This study evaluated factors impacting QTc interval in a phase 3 trial of cabozantinib in progressive, metastatic, medullary thyroid cancer (MTC).
Methods
Electrocardiogram (12-lead ECG) measurements were obtained at screening, and at pre-dose, and 2, 4, and 6 h post-dose on Days 1 and 29 in a phase 3 study in patients with MTC treated with cabozantinib (140 mg/day). Central tendency analyses were conducted on baseline-corrected QTc values. Linear and nonlinear mixed-effects models were used to evaluate potential factors affecting the QTc interval, including serum electrolytes, patient demographics, and cabozantinib concentration.
Results
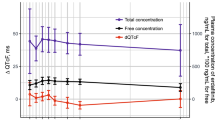
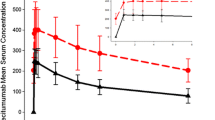
Central tendency analysis showed that oral cabozantinib (140 mg/day) produced a 10–15 ms increase in delta–delta Fridericia corrected QT (∆∆QTcF) and delta–delta study-specific corrected QT (∆∆QTcS) on Day 29, but not on Day 1. Further analysis showed that QTcS provided a slightly more accurate QT correction than QTcF. Mixed-effects models evaluating serum electrolytes, age, sex, and cabozantinib concentration showed that decreased serum calcium and potassium could explain the majority of cabozantinib treatment-associated QTcS prolongation observed in this study.
Conclusions
Cabozantinib treatment prolongs the ∆∆QTcF interval by 10–15 ms. There was the absence of a strong relationship between cabozantinib concentration and QTcS prolongation. Cabozantinib treatment effects on serum calcium and potassium best explain the QTcS prolongation observed in this study.




Similar content being viewed by others
References
Guideline IHT (2005) The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs E14. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002879.pdf. Accessed 30 Dec 2016
Yap YG, Camm AJ (2003) Drug induced QT prolongation and torsades de pointes. Heart 89(11):1363–1372
Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A (2000) The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications. Cardiovasc Res 47(2):219–233
Grant AO (2009) Cardiac ion channels. Circ Arrhythm Electrophysiol 2(2):185–194. doi:10.1161/CIRCEP.108.789081
Strevel EL, Ing DJ, Siu LL (2007) Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 25(22):3362–3371. doi:10.1200/JCO.2006.09.6925
Brell JM (2010) Prolonged QTc interval in cancer therapeutic drug development: defining arrhythmic risk in malignancy. Prog Cardiovasc Dis 53(2):164–172
Curigliano G, Spitaleri G, De Braud F, Cardinale D, Cipolla C, Civelli M, Colombo N, Colombo A, Locatelli M, Goldhirsch A (2009) QTc prolongation assessment in anticancer drug development: clinical and methodological issues. ecancermedicalscience 3:130
Salvi V, Karnad DR, Panicker GK, Kothari S (2010) Update on the evaluation of a new drug for effects on cardiac repolarization in humans: issues in early drug development. Br J Pharmacol 159(1):34–48
Sarapa N, Britto MR (2008) Challenges of characterizing proarrhythmic risk due to QTc prolongation induced by nonadjuvant anticancer agents. Expert Opin Drug Saf 7(3):305–318
Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B (2011) Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10(12):2298–2308
Capp C, Wajner SM, Siqueira DR, Brasil BA, Meurer L, Maia AL (2010) Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid 20(8):863–871
Cassinelli G, Favini E, Degl’Innocenti D, Salvi A, De Petro G, Pierotti MA, Zunino F, Borrello MG, Lanzi C (2009) RET/PTC1-driven neoplastic transformation and proinvasive phenotype of human thyrocytes involve Met induction and β-catenin nuclear translocation. Neoplasia 11(1):10–21
Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M (2002) ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Can Res 62(24):7284–7290
COMETRIQ(R) [package insert] (2016) Exelixis Inc., South San Francisco. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203756s002lbl.pdf. Accessed 30 Dec 2016
US Food and Drug Administration CfDEaR (2012) Approval package for application number 203756Orig1s000 for cabozantinib (COMETRIQ(R)). Clin Pharmacol Biopharm Rev(s). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203756Orig1s000ClinPharmR.pdf. Accessed 30 Dec 2016
Miles D, Jumbe NL, Lacy S, Nguyen L (2016) Population pharmacokinetic model of cabozantinib in patients with medullary thyroid carcinoma and its application to an exposure-response analysis. Clin Pharmacokinet 55(1):93–105. doi:10.1007/s40262-015-0295-x
Nguyen L, Holland J, Miles D, Engel C, Benrimoh N, O’Reilly T, Lacy S (2015) Pharmacokinetic (PK) drug interaction studies of cabozantinib: effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol 55(9):1012–1023. doi:10.1002/jcph.510
Nguyen L, Holland J, Mamelok R, Laberge MK, Grenier J, Swearingen D, Armas D, Lacy S (2015) Evaluation of the effect of food and gastric pH on the single-dose pharmacokinetics of cabozantinib in healthy adult subjects. J Clin Pharmacol 55(11):1293–1302. doi:10.1002/jcph.526
Lacy SA, Miles DR, Nguyen LT (2017) Clinical pharmacokinetics and pharmacodynamics of cabozantinib. Clin Pharmacokinet. 56(5):477–491. doi:10.1007/s40262-016-0461-9
Sanguinetti MC, Jiang C, Curran ME, Keating MT (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: hERG encodes the IKr potassium channel. Cell 81(2):299–307
Sanguinetti MC, Tristani-Firouzi M (2006) hERG potassium channels and cardiac arrhythmia. Nature 440(7083):463–469. doi:10.1038/nature04710
Wible BA, Hawryluk P, Ficker E, Kuryshev YA, Kirsch G, Brown AM (2005) HERG-Lite: a novel comprehensive high-throughput screen for drug-induced hERG risk. J Pharmacol Toxicol Methods 52(1):136–145. doi:10.1016/j.vascn.2005.03.008
Kiehn J, Lacerda AE, Wible B, Brown AM (1996) Molecular physiology and pharmacology of HERG single-channel currents and block by dofetilide. Circulation 94(10):2572–2579
Mohammad S, Zhou Z, Gong Q, January CT (1997) Blockage of the HERG human cardiac K + channel by the gastrointestinal prokinetic agent cisapride. Am J Physiol-Heart Circ Physiol 273(5):H2534–H2538
Danker T, Moller C (2014) Early identification of hERG liability in drug discovery programs by automated patch clamp. Front Pharmacol 5:203. doi:10.3389/fphar.2014.00203
US Food and Drug Administration CfDEaR (2012) Approval package for application number 203756Orig1s000 for cabozantinib (COMETRIQ(R)). Pharmacol Rev. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203756Orig1s000PharmR.pdf. Accessed 30 Dec 2016
US Food and Drug Administration CfDEaR (2005) Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073153.pdf. Accessed 30 Dec 2016
Bello CL, Mulay M, Huang X, Patyna S, Dinolfo M, Levine S, Van Vugt A, Toh M, Baum C, Rosen L (2009) Electrocardiographic characterization of the QTc interval in patients with advanced solid tumors: pharmacokinetic- pharmacodynamic evaluation of sunitinib. Clin Cancer Res 15(22):7045–7052. doi:10.1158/1078-0432.CCR-09-1521
Sonnichsen D, Dorer DJ, Cortes J, Talpaz M, Deininger MW, Shah NP, Kantarjian HM, Bixby D, Mauro MJ, Flinn IW, Litwin J, Turner CD, Haluska FG (2013) Analysis of the potential effect of ponatinib on the QTc interval in patients with refractory hematological malignancies. Cancer Chemother Pharmacol 71(6):1599–1607. doi:10.1007/s00280-013-2160-7
Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31(29):3639–3646
Genovesi S, Dossi C, Vigano MR, Galbiati E, Prolo F, Stella A, Stramba-Badiale M (2008) Electrolyte concentration during haemodialysis and QT interval prolongation in uraemic patients. Europace 10(6):771–777. doi:10.1093/europace/eun028
Hosmane B, Locke C, Morris D (2006) QT interval correction for heart rate. J Appl Res Clin Exp Ther 6(4):288–299
Tornøe CW, Garnett CE, Wang Y, Florian J, Li M, Gobburu JV (2011) Creation of a knowledge management system for QT analyses. J Clin Pharmacol 51(7):1035–1042
Honig PK, Wortham DC, Zamani K, Conner DP, Mullin JC, Cantilena LR (1993) Terfenadine-ketoconazole interaction: pharmacokinetic and electrocardiographic consequences. JAMA 269(12):1513–1518
Afshinnia F, Doshi H, Rao PS (2012) The effect of different dialysate magnesium concentrations on QTc dispersion in hemodialysis patients. Ren Fail 34(4):408–412
Garnett CE, Zhu H, Malik M, Fossa AA, Zhang J, Badilini F, Li J, Darpo B, Sager P, Rodriguez I (2012) Methodologies to characterize the QT/corrected QT interval in the presence of drug-induced heart rate changes or other autonomic effects. Am Heart J 163(6):912–930. doi:10.1016/j.ahj.2012.02.023
Malik M, Färbom P, Batchvarov V, Hnatkova K, Camm A (2002) Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart 87(3):220–228
Batchvarov VN, Ghuran A, Smetana P, Hnatkova K, Harries M, Dilaveris P, Camm AJ, Malik M (2002) QT-RR relationship in healthy subjects exhibits substantial intersubject variability and high intrasubject stability. Am J Physiol-Heart Circ Physiol 282(6):H2356–H2363
Ghatalia P, Je Y, Kaymakcalan M, Sonpavde G, Choueiri T (2015) QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br J Cancer 112(2):296–305
US Food and Drug Administration CfDEaR (August 2010) Application number 022405Orig1s000 for vandetanib (CAPRELSA(R)). Pharmacol Rev(s). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022405Orig1s000PharmR.pdf. Accessed 30 Dec 2016
Chau NG, Haddad RI (2013) Vandetanib for the treatment of medullary thyroid cancer. Clin Cancer Res 19(3):524–529. doi:10.1158/1078-0432.CCR-12-2353
ICLUSIG(R) [package insert] (2016) ARIAD Pharmaceuticals Inc., Cambridge. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203469s022lbl.pdf. Accessed 30 Dec 2016
PROLIA(R) [summary of product characteristics] (2017) AMGEN LIMITED, Cambridge, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002173/WC500110381.pdf. Accessed 29 Apr 2017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest and source of funding
These analyses were funded by Exelixis, Inc. Steve Lacy and Steve Milwee are full-time employees and stockholders of Exelixis Inc. Dale Miles, Yifah Yaron, and Linh Nguyen were full-time employees and stockholders of Exelixis, Inc. when the work was performed. Dale Miles is currently employed Genentech Inc. and Linh Nguyen is currently employed by Pfizer Inc. David Russ Wada is a full-time employee of Certara, which was contracted to perform some analyses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miles, D.R., Lacy, S.A., Wada, D.R. et al. Assessment of cabozantinib treatment on QT interval in a phase 3 study in medullary thyroid cancer: evaluation of indirect QT effects mediated through treatment-induced changes in serum electrolytes. Cancer Chemother Pharmacol 80, 295–306 (2017). https://doi.org/10.1007/s00280-017-3349-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3349-y