Abstract
Purpose
The aim of this study was to evaluate safety and toxicity of chronomodulated capecitabine administered in the morning and at noon according to a specific time schedule (Brunch Regimen: Breakfast and Lunch) as a part of first-line XELOX chemotherapy in patients with metastatic colorectal cancer.
Methods
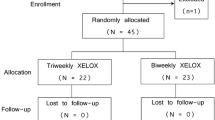
A total of 30 treatment-naïve colorectal cancer patients with metastatic disease were included. Oxaliplatin 130 mg/m2 on day 1 plus chronomodulated oral capecitabine 2000 mg/m2 per day were administered (50 % dose at 8:00 a.m. and 50 % dose at 12:00 noon on days 1–14, every 21 days). All adverse events, treatment responses and survival were evaluated. In addition, pharmacokinetic profile of capecitabine was examined in a subset of 5 patients.
Results
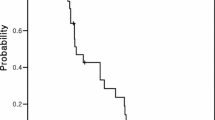
Median age was 57.1 years (range 32–77 years). Median follow-up was 19 months (range 3–36 months). Three patients (10 %) had complete response, 13 patients (43.3 %) had partial response and 4 patients (13.3 %) had stabile disease. Ten patients had progressive disease at their first evaluation (33.3 %). The median progression-free survival (PFS) was 10 months (range 2–36 months). There were no grade 4 toxicities. One patient (3.3 %) had grade 3 neutropenia. Hand-foot syndrome developed in three patients (10 %): 6.6 %, grade 1 and 3.3 %, grade 2.
Conclusions
Chronomodulated XELOX seems to represent a promising therapeutic option in the first-line treatment of metastatic colorectal carcinoma due to good tumor control and favorable toxicity profile. Phase III randomized trials are required to assess the actual clinical efficacy and side effect profile of this regimen.


Similar content being viewed by others
References
Ragnhammar P, Hafstrom L, Nygren P, Glimelius B, Care SB-gSCoTAiH (2001) A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol 40(2–3):282–308
Kohne-Wompner CH, Schmoll HJ, Harstrick A, Rustum YM (1992) Chemotherapeutic strategies in metastatic colorectal cancer: an overview of current clinical trials. Semin Oncol 19(2 Suppl 3):105–125
Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D (1993) Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 306(6880):752–755
Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P (1998) Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352(9138):1413–1418. doi:10.1016/S0140-6736(98)02309-5
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355(9209):1041–1047
Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG, Le Bail N, Haller DG (2003) Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil–leucovorin: interim results of a phase III trial. J Clin Oncol 21(11):2059–2069. doi:10.1200/JCO.2003.11.126
Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B (2000) Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 45(4):291–297. doi:10.1007/s002800050043
Reigner B, Blesch K, Weidekamm E (2001) Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet 40(2):85–104. doi:10.2165/00003088-200140020-00002
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Saltz L (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26(12):2006–2012. doi:10.1200/JCO.2007.14.9898
Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, Gollins S, Siu LL, Laguerre S, Cunningham D (2008) Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol 19(10):1720–1726. doi:10.1093/annonc/mdn370
Diaz-Rubio E, Evans TR, Tabemero J, Cassidy J, Sastre J, Eatock M, Bisset D, Regueiro P, Baselga J (2002) Capecitabine (Xeloda) in combination with oxaliplatin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumors. Ann Oncol 13(4):558–565
Levi F (1997) Chronopharmacology of anticancer agents. In: Redfern PH, Lemmer B (eds) Handbook of experimental pharmacology: physiology and pharmacology of biological rhythms. Springer, Berlin, pp 299–331
Levi FA, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, Chollet P, Garufi C, Itzhaki M, Dogliotti L et al (1994) Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst 86(21):1608–1617
Levi F, Zidani R, Misset JL (1997) Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350(9079):681–686
Bajetta E, Pietrantonio F, Buzzoni R, Ferrario E, Valvo F, Mariani L, Dotti KF, Biondani P, Formisano B, Gevorgyan A, Grassi P, Di Bartolomeo M (2014) Chronomodulated capecitabine and adjuvant radiation in intermediate-risk to high-risk rectal cancer: a phase II study. Am J Clin Oncol 37(6):545–549. doi:10.1097/COC.0b013e31827ecd1d
Akgun Z, Saglam S, Yucel S, Gural Z, Balik E, Cipe G, Yildiz S, Kilickap S, Okyar A, Kaytan-Saglam E (2014) Neoadjuvant chronomodulated capecitabine with radiotherapy in rectal cancer: a phase II brunch regimen study. Cancer Chemother Pharmacol 74(4):751–756. doi:10.1007/s00280-014-2558-x
Kennedy MF, Tutton PJ, Barkla DH (1985) Comparison of the circadian variation in cell proliferation in normal and neoplastic colonic epithelial cells. Cancer Lett 28(2):169–175
Delord JP, Umlil A, Guimbaud R, Gregoire N, Lafont T, Canal P, Bugat R, Chatelut E (2003) Population pharmacokinetics of oxaliplatin. Cancer Chemother Pharmacol 51(2):127–131. doi:10.1007/s00280-002-0550-3
Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J, Price T, Lim R, Van Cutsem E, Park YS, McKendrick J, Topham C, Soler-Gonzalez G, de Braud F, Hill M, Sirzen F, Haller DG (2007) Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1864 patients. J Clin Oncol 25(1):102–109. doi:10.1200/JCO.2006.08.1075
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Rittweger K, Gilberg F, Saltz L (2011) XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer 105(1):58–64. doi:10.1038/bjc.2011.201
Santini D, Vincenzi B, Schiavon G, Di Seri M, Virzi V, Spalletta B, Caricato M, Coppola R, Tonini G (2007) Chronomodulated administration of oxaliplatin plus capecitabine (XELOX) as first line chemotherapy in advanced colorectal cancer patients: phase II study. Cancer Chemother Pharmacol 59(5):613–620. doi:10.1007/s00280-006-0302-x
Qvortrup C, Jensen BV, Fokstuen T, Nielsen SE, Keldsen N, Glimelius B, Bjerregaard B, Mejer J, Larsen FO, Pfeiffer P (2010) A randomized study comparing short-time infusion of oxaliplatin in combination with capecitabine XELOX(30) and chronomodulated XELOX(30) as first-line therapy in patients with advanced colorectal cancer. Ann Oncol 21(1):87–91. doi:10.1093/annonc/mdp272
Muggia FM, Wu X, Spicer D, Groshen S, Jeffers S, Leichman CG, Leichman L, Chan KK (1996) Phase I and pharmacokinetic study of oral UFT, a combination of the 5-fluorouracil prodrug tegafur and uracil. Clin Cancer Res 2(9):1461–1467
Etienne-Grimaldi MC, Francois E, Cardot JM, Renee N, Douillard JY, Gamelin E, Bennouna J, Chateau Y, Milano G (2007) A clinical pharmacokinetic analysis of tegafur-uracil (UFT) plus leucovorin given in a new twice-daily oral administration schedule. Clin Pharmacokinet 46(11):953–963. doi:10.2165/00003088-200746110-00003
Ikeda K, Yoshisue K, Matsushima E, Nagayama S, Kobayashi K, Tyson CA, Chiba K, Kawaguchi Y (2000) Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro. Clin Cancer Res 6(11):4409–4415
Milano G, Ferrero JM, Francois E (2004) Comparative pharmacology of oral fluoropyrimidines: a focus on pharmacokinetics, pharmacodynamics and pharmacomodulation. Br J Cancer 91(4):613–617. doi:10.1038/sj.bjc.6601973
Farkouh A, Ettlinger D, Schueller J, Georgopoulos A, Scheithauer W, Czejka M (2010) A rapid and simple HPLC assay for quantification of capecitabine for drug monitoring purposes. Anticancer Res 30(12):5207–5211
Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S, Jansen G, van Groeningen CJ, Pinedo HM (2002) Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta 1587(2–3):194–205
Midgley R, Kerr DJ (2009) Capecitabine: have we got the dose right? Nat Clin Pract Oncol 6(1):17–24. doi:10.1038/ncponc1240
Harris BE, Song RL, He YJ, Soong SJ, Diasio RB (1988) Circadian rhythm of rat liver dihydropyrimidine dehydrogenase. Possible relevance to fluoropyrimidine chemotherapy. Biochem Pharmacol 37(24):4759–4762
Brown WR (1991) A review and mathematical analysis of circadian rhythms in cell proliferation in mouse, rat, and human epidermis. J Invest Dermatol 97(2):273–280
Lincoln DW 2nd, Hrushesky WJ, Wood PA (2000) Circadian organization of thymidylate synthase activity in normal tissues: a possible basis for 5-fluorouracil chronotherapeutic advantage. Int J Cancer 88(3):479–485
Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB, Hrushesky WJ, Ben-David Y (2001) Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol 158(5):1793–1801. doi:10.1016/S0002-9440(10)64135-1
Burns ER, Bagwell CB, Hinson WG, Pipkin JL Jr, Hudson JL (1983) Preparation and stability of sixteen murine tissues and organs for flow cytometric cell cycle analysis. Cytometry 4(2):150–160. doi:10.1002/cyto.990040208
Acknowledgments
We deeply express our gratitude to A. Nur Sozudogru M.D. for his grammar and spelling revision of the text.
Funding
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University. Project number is ONAP-25139.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilancı, K.N., Saglam, S., Okyar, A. et al. Chronomodulated oxaliplatin plus Capecitabine (XELOX) as a first line chemotherapy in metastatic colorectal cancer: A Phase II Brunch regimen study. Cancer Chemother Pharmacol 78, 143–150 (2016). https://doi.org/10.1007/s00280-016-3067-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3067-x