Abstract
Purpose
This Phase I dose-escalation study (GASTANA) evaluated the safety, tolerability, pharmacokinetics and preliminary antitumor activity of cabazitaxel in Asian patients with advanced gastric adenocarcinoma failing two prior chemotherapy regimens.
Methods
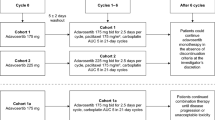
Cabazitaxel safety/tolerability was determined using a standard 3 + 3 dose-escalation design based on dose-limiting toxicities (DLTs) in Cycle 1. Three dose levels (DL) were planned: 20, 25 and 15 mg/m2 (DL 1, DL 2 and DL −1).
Results
Fifteen patients were evaluable for DLTs. At DL 1, no DLTs occurred in three patients. At DL 2, four patients were enrolled (one patient discontinued), with only one DLT observed [Grade 4 febrile neutropenia (FN)]; however, all four patients experienced FN, hence three more patients were enrolled at DL 1 who experienced two DLTs (Grade 4 neutropenia >7 days). In response, DL −1 was opened, with no DLTs observed in six patients. In the total population (n = 16), frequent Grade 3/4 toxicities included neutropenia (63 %) and FN (38 %), best overall responses included one partial response (6.3 %; DL −1) and eight stable disease (50 %), and median progression-free survival was 83 days.
Conclusions
No unexpected safety findings were observed. Significant toxicities included neutropenia and FN, potentially due to patients being heavily pretreated and the accumulated toxicity of prior taxane therapy.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2013) GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer, Lyon. http://globocan.iarc.fr
Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, Park SR, Fujii M, Kang YK, Chen LT (2013) Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol 14:e535–e547
Van Cutsem E, Dicato M, Geva R, Arber N, Bang Y, Benson A, Cervantes A, Diaz-Rubio E, Ducreux M, Glynne-Jones R, Grothey A, Haller D, Haustermans K, Kerr D, Nordlinger B, Marshall J, Minsky BD, Kang YK, Labianca R, Lordick F, Ohtsu A, Pavlidis N, Roth A, Rougier P, Schmoll HJ, Sobrero A, Tabernero J, Van de Velde C, Zalcberg J (2011) The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol 22(Suppl 5):v1–v9
Ford H, Marshall A, Wadsley J, Coxon FY, Mansoor W, Bridgewater JA, Madhusudan S, Falk S, Middleton GW, Swinson D, Chau I, Thompson J, Blazeby JM, Cunningham D, Kareclas P, Dunn JA (2012) Cougar-02: a randomized phase III study of docetaxel versus active symptom control in advanced esophagogastric adenocarcinoma. J Clin Oncol 30. abstract LBA4
Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY, Kang WK, Park SH (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30:1513–1518
Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P (2011) Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 47:2306–2314
National Comprehensive Cancer Network: Gastric Cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Gastric Cancer (Version 1.2014). http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
Vrignaud P, Sémiond D, Lejeune P, Bouchard H, Calvet L, Combeau C, Riou J-F, Commercon A, Lavelle F, Bissery M-C (2013) Preclinical antitumor activity of cabazitaxel, a semi-synthetic taxane active in taxane-resistant tumors. Clin Cancer Res 19:2973–2983
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376:1147–1154
National Comprehensive Cancer Network: Prostate Cancer. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer (Version 2.2014). http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
Pean E, Demolis P, Moreau A, Hemmings RJ, O’Connor D, Brown D, Shepard T, Abadie E, Pignatti F (2012) The European Medicines Agency review of cabazitaxel (Jevtana(R)) for the treatment of hormone-refractory metastatic prostate cancer: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist 17:543–549
Sanofi (2011) JEVTANA® (cabazitaxel) injection, summary of product characteristics. EMA, London, UK
Sanofi U.S. LLC (2013) JEVTANA® (cabazitaxel) injection, prescribing information. FDA, USA
National Cancer Institute (2010) Common terminology criteria for adverse events (CTCAE) version 4.03. http://evs.nci.nih.gov/ftp1/CTCAE/About.html
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2:228–247
Mita AC, Denis LJ, Rowinsky EK, de Bono JS, Goetz AD, Ochoa L, Forouzesh B, Beeram M, Patnaik A, Molpus K, Semiond D, Besenval M, Tolcher AW (2009) Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res 15:723–730
Dieras V, Lortholary A, Laurence V, Delva R, Girre V, Livartowski A, Assadourian S, Semiond D, Pierga JY (2013) Cabazitaxel in patients with advanced solid tumours: results of a phase I and pharmacokinetic study. Eur J Cancer 49:25–34
Yano R, Konno A, Watanabe K, Tsukamoto H, Kayano Y, Ohnaka H, Goto N, Nakamura T, Masada M (2013) Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol 18:96–104
Fumoleau P, Trigo JM, Isambert N, Semiond D, Gupta S, Campone M (2013) Phase I dose-finding study of cabazitaxel administered weekly in patients with advanced solid tumours. BMC Cancer 13:460
Mukai H, Takahashi S, Nozawa M, Onozawa Y, Miyazaki J, Ohno K, Suzuki K (2014) Phase I dose-escalation and pharmacokinetic study (TED 11576) of cabazitaxel in Japanese patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 73:703–710
Kang EJ, Im SA, Oh DY, Han SW, Kim JS, Choi IS, Kim JW, Kim YJ, Kim JH, Kim TY, Lee JS, Bang YJ, Lee KW (2013) Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: treatment outcomes and a prognostic model to predict survival. Gastric Cancer 16:581–589
Kang YK, Muro K, Ryu MH, Yasui H, Nishina T, Ryoo BY, Kamiya Y, Akinaga S, Boku N (2014) A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs 32:355–361
Lee MJ, Hwang IG, Jang JS, Choi JH, Park BB, Chang MH, Kim ST, Park SH, Kang MH, Kang JH (2012) Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res Treat 44:235–241
Acknowledgments
This study was funded by Sanofi. The authors received editorial support from Paul Scutt of MediTech Media Ltd, funded by Sanofi. This study was funded by Sanofi.
Conflict of interest
Yoon-Koo Kang has a consultancy/advisory role with Sanofi, Roche, Daiho and Novartis and has received funding from Sanofi, Roche, Jeil, Novartis and Bayer. Lin Shen has received remuneration from Roche, Novartis and Amgen, funding from Celgene, Taiho, Heng Rui, Sanofi and Roche, and has a consultant/advisory role with Roche and Novartis. Yu Zhou, Jooyun Lee and Chenlu Wei are employees of Sanofi. Baek-Yeol Ryoo, Shinkyo Yoon and Min-Hee Ryu have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, YK., Ryoo, BY., Yoon, S. et al. A Phase I study of cabazitaxel in patients with advanced gastric cancer who have failed prior chemotherapy (GASTANA). Cancer Chemother Pharmacol 75, 309–318 (2015). https://doi.org/10.1007/s00280-014-2638-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2638-y