Abstract
Purpose
Metastatic melanoma is considered one of the most aggressive malignant tumours, representing the deadliest form of skin cancer. Melanoma progression is associated with the abrogation of normal controls that limit cell proliferation, migration, and invasion, eventually leading to metastasis. Based on the variety of cellular mechanisms involved in metastatic progression, we aimed to evaluate the effect of inosine (50 μM) and of the combination of Cl-IB-MECA (10 μM) with paclitaxel (10 ng/mL) on several stages of melanoma progression.
Methods
Proliferation, migration, adhesion, invasion, and colony formation assays were performed on human C32 and A375 metastatic melanoma cells. Levels of ERK1/2 were also determined using an ELISA kit. Moreover, mouse aortic rings were treated with vascular endothelial growth factor in order to assess the microvessel sprouting (an indicator of angiogenesis) in the presence of the referred compounds.
Results
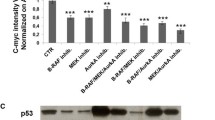
We demonstrate that inosine induced, through A3 adenosine receptor activation, proliferation, migration, adhesion, and invasion on C32 and A375 melanoma cells, although with dissimilar importance on the two melanoma cell lines. Inosine also increased colony formation on A375 cells. Levels of ERK1/2 were increased after inosine exposure and that increase was dependent on A3 adenosine receptor activation in both cell lines. Moreover, microvessel sprouting stimulated by inosine was decreased by the combination of Cl-IB-MECA with paclitaxel.
Conclusions
Cl-IB-MECA combined with paclitaxel was able to impair almost all of the referred metastatic related mechanisms induced by inosine, making this approach a valuable tool for combinatory therapy against metastatic melanoma.






Similar content being viewed by others
Abbreviations
- AR:
-
Adenosine receptor
- BME:
-
Basement membrane extract
- BSA:
-
Bovine serum albumin
- Cl-IB-MECA:
-
2-Chloro-N(6)-(3-iodobenzyl)-adenosine-5′-N-methyl-uronamide
- CTR:
-
Control
- DMSO:
-
Dimethyl sulfoxide
- DMEM-HG:
-
Dulbecco’s modified Eagle’s medium–high glucose
- ECM:
-
Extracellular matrix
- ERK:
-
Extracellular signal-regulated kinase
- FBS:
-
Foetal bovine serum
- INO:
-
Inosine
- MAPK:
-
Mitogen-activated protein kinase
- MRE3008F20:
-
N-[2-(2-furanyl)-8-propyl-8H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine-5-yl]-N′-(4-methoxyphenyl)urea
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- Opti-MEM:
-
Opti-minimal essential medium
- PBS:
-
Phosphate-buffered saline
- PI3K:
-
Phosphatidylinositol 3-kinase
- PXT:
-
Paclitaxel
- ROS:
-
Reactive oxygen species
- VEGF:
-
Vascular endothelial growth factor
References
Bandarchi B, Ma L, Navab R, Seth A, Rasty G (2010) From melanocyte to metastatic malignant melanoma. Dermatol Res Pract 2010. doi:10.1155/2010/583748
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
Bhatia S, Tykodi SS, Thompson JA (2009) Treatment of metastatic melanoma: an overview. Oncology 23(6):488–496
Soares AS, Costa VM, Diniz C, Fresco P (2013) Potentiation of cytotoxicity of paclitaxel in combination with Cl-IB-MECA in human C32 metastatic melanoma cells: a new possible therapeutic strategy for melanoma. Biomed Pharmacother 67(8):777–789
Soares AS, Costa VM, Diniz C, Fresco P (2014) Combination of Cl-IB-MECA with paclitaxel is a highly effective cytotoxic therapy causing mTOR-dependent autophagy and mitotic catastrophe on human melanoma cells. J Cancer Res Clin Oncol. doi:10.1007/s00432-014-1645-z
Madonna G, Ullman CD, Gentilcore G, Palmieri G, Ascierto PA (2012) NF-kappaB as potential target in the treatment of melanoma. J Transl Med 10:53. doi:10.1186/1479-5876-10-53
Haass NK, Herlyn M (2005) Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Investig Dermatol Symp Proc 10(2):153–163
Haass NK, Smalley KS, Li L, Herlyn M (2005) Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res Spons Eur Soc Pigment Cell Res Int Pigment Cell Soc 18(3):150–159
Attoub S, Arafat K, Gelaude A, Al Sultan MA, Bracke M, Collin P, Takahashi T, Adrian TE, De Wever O (2013) Frondoside a suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis. PLoS One 8(1):e53087
Huang YW, Baluna R, Vitetta ES (1997) Adhesion molecules as targets for cancer therapy. Histol Histopathol 12(2):467–477
Yamazoe Y, Tsubaki M, Matsuoka H, Satou T, Itoh T, Kusunoki T, Kidera Y, Tanimori Y, Shoji K, Nakamura H, Ogaki M, Nishiura S, Nishida S (2009) Dimethylfumarate inhibits tumor cell invasion and metastasis by suppressing the expression and activities of matrix metalloproteinases in melanoma cells. Cell Biol Int 33(10):1087–1094
Mori S, Chang JT, Andrechek ER, Matsumura N, Baba T, Yao G, Kim JW, Gatza M, Murphy S, Nevins JR (2009) Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene 28(31):2796–2805
Abdollahi A, Folkman J (2010) Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Update Rev Comment Antimicrob Anticancer Chemother 13(1–2):16–28
Spychala J (2000) Tumor-promoting functions of adenosine. Pharmacol Ther 87(2–3):161–173
Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ (2011) CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 71(8):2892–2900
Di Virgilio F (2012) Purines, purinergic receptors, and cancer. Cancer Res 72(21):5441–5447
Soares AS, Costa VM, Diniz C, Fresco P (2014) Inosine strongly enhances human C32 melanoma cells proliferation through PLC-PKC-MEK1/2-ERK1/2 and PI3K pathways. Basic Clin Pharmacol Toxicol. doi:10.1111/bcpt.12280
Vannoni D, Bernini A, Carlucci F, Civitelli S, Di Pietro MC, Leoncini R, Rosi F, Tabucchi A, Tanzini G, Marinello E (2004) Enzyme activities controlling adenosine levels in normal and neoplastic tissues. Med Oncol 21(2):187–195
Linden J (2001) Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 41:775–787
Pirincci N, Gecit I, Gunes M, Yuksel MB, Kaba M, Tanik S, Demir H, Aslan M (2012) Serum adenosine deaminase, catalase and carbonic anhydrase activities in patients with bladder cancer. Clinics 67(12):1443–1446
Aghaei M, Karami-Tehrani F, Salami S, Atri M (2005) Adenosine deaminase activity in the serum and malignant tumors of breast cancer: the assessment of isoenzyme ADA1 and ADA2 activities. Clin Biochem 38(10):887–891
Zanini D, Schmatz R, Pelinson LP, Pimentel VC, da Costa P, Cardoso AM, Martins CC, Schetinger CC, Baldissareli J, do Carmo Araujo M, Oliveira L, Chiesa J, Morsch VM, Leal DB, Schetinger MR (2013) Ectoenzymes and cholinesterase activity and biomarkers of oxidative stress in patients with lung cancer. Mol Cell Biochem 374(1–2):137–148
Shafy A, Molinie V, Cortes-Morichetti M, Hupertan V, Lila N, Chachques JC (2012) Comparison of the effects of adenosine, inosine, and their combination as an adjunct to reperfusion in the treatment of acute myocardial infarction. ISRN Cardiol 2012:326809
Schram KH (1998) Urinary nucleosides. Mass Spectrom Rev 17(3):131–251
Gessi S, Varani K, Merighi S, Cattabriga E, Avitabile A, Gavioli R, Fortini C, Leung E, Mac Lennan S, Borea PA (2004) Expression of A3 adenosine receptors in human lymphocytes: up-regulation in T cell activation. Mol Pharmacol 65(3):711–719
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2(2):329–333
Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK (2007) MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res 5(4):309–320
Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D (2009) LKB1 is required for adiponectin-mediated modulation of AMPK-S6 K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 28(29):2621–2633
Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K (2012) Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 7(1):89–104
Jin X, Shepherd RK, Duling BR, Linden J (1997) Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Investig 100(11):2849–2857
Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ (2010) Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA 107(4):1547–1552
Merighi S, Mirandola P, Milani D, Varani K, Gessi S, Klotz KN, Leung E, Baraldi PG, Borea PA (2002) Adenosine receptors as mediators of both cell proliferation and cell death of cultured human melanoma cells. J Invest Dermatol 119(4):923–933
Wolber C, Fozard JR (2005) The receptor mechanism mediating the contractile response to adenosine on lung parenchymal strips from actively sensitised, allergen-challenged Brown Norway rats. Naunyn-Schmiedeberg’s Arch Pharmacol 371(2):158–168
Mlejnek P, Dolezel P, Frydrych I (2013) Effects of synthetic A3 adenosine receptor agonists on cell proliferation and viability are receptor independent at micromolar concentrations. J Physiol Biochem 69(3):405–417
Mervic L (2012) Time course and pattern of metastasis of cutaneous melanoma differ between men and women. PLoS One 7(3):e32955
Ahmed AA, Wang X, Lu Z, Goldsmith J, Le XF, Grandjean G, Bartholomeusz G, Broom B, Bast RC Jr (2011) Modulating microtubule stability enhances the cytotoxic response of cancer cells to Paclitaxel. Cancer Res 71(17):5806–5817
Tran TA, Gillet L, Roger S, Besson P, White E, Le Guennec JY (2009) Non-anti-mitotic concentrations of taxol reduce breast cancer cell invasiveness. Biochem Biophys Res Commun 379(2):304–308
Ohkawa Y, Miyazaki S, Hamamura K, Kambe M, Miyata M, Tajima O, Ohmi Y, Yamauchi Y, Furukawa K, Furukawa K (2010) Ganglioside GD3 enhances adhesion signals and augments malignant properties of melanoma cells by recruiting integrins to glycolipid-enriched microdomains. J Biol Chem 285(35):27213–27223
Sadej R, Spychala J, Skladanowski AC (2006) Expression of ecto-5′-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res 16(3):213–222
Wang F, Cao Y, Liu HY, Xu SF, Han R (2003) Anti-invasion and anti-angiogenesis effect of taxol and camptothecin on melanoma cells. J Asian Nat Prod Res 5(2):121–129
Locatelli C, Leal PC, Yunes RA, Nunes RJ, Creczynski-Pasa TB (2009) Gallic acid ester derivatives induce apoptosis and cell adhesion inhibition in melanoma cells: the relationship between free radical generation, glutathione depletion and cell death. Chem Biol Interact 181(2):175–184
Sallam AA, Ramasahayam S, Meyer SA, El Sayed KA (2010) Design, synthesis, and biological evaluation of dibromotyrosine analogues inspired by marine natural products as inhibitors of human prostate cancer proliferation, invasion, and migration. Bioorgan Med Chem 18(21):7446–7457
Stemmer SM, Benjaminov O, Medalia G, Ciuraru NB, Silverman MH, Bar-Yehuda S, Fishman S, Harpaz Z, Farbstein M, Cohen S, Patoka R, Singer B, Kerns WD, Fishman P (2013) CF102 for the treatment of hepatocellular carcinoma: a phase I/II, open-label, dose-escalation study. Oncologist 18(1):25–26
Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Borea PA (2005) A3 adenosine receptor activation inhibits cell proliferation via phosphatidylinositol 3-kinase/Akt-dependent inhibition of the extracellular signal-regulated kinase 1/2 phosphorylation in A375 human melanoma cells. J Biol Chem 280(20):19516–19526
Conner SR, Scott G, Aplin AE (2003) Adhesion-dependent activation of the ERK1/2 cascade is by-passed in melanoma cells. J Biol Chem 278(36):34548–34554
Romanchikova N, Trapencieris P, Zemitis J, Turks M (2013) A novel matrix metalloproteinase-2 inhibitor triazolylmethyl aziridine reduces melanoma cell invasion, angiogenesis and targets ERK1/2 phosphorylation. J Enzyme Inhib Med Chem. doi:10.3109/14756366.2013.855207
Bar-Yehuda S, Madi L, Silberman D, Gery S, Shkapenuk M, Fishman P (2005) CF101, an agonist to the A3 adenosine receptor, enhances the chemotherapeutic effect of 5-fluorouracil in a colon carcinoma murine model. Neoplasia 7(1):85–90
Sung SY, Hsieh CL, Wu D, Chung LW, Johnstone PA (2007) Tumor microenvironment promotes cancer progression, metastasis, and therapeutic resistance. Curr Probl Cancer 31(2):36–100
Kim GD, Oh J, Jeong LS, Lee SK (2013) Thio-Cl-IB-MECA, a novel A(3) adenosine receptor agonist, suppresses angiogenesis by regulating PI3K/AKT/mTOR and ERK signaling in endothelial cells. Biochem Biophys Res Commun 437(1):79–86
Acknowledgments
Work was funded by FEDER through the Program of Operational Competitiveness Factors—COMPETE and National Funds through FCT—Foundation for Science and Technology. ASS and VMC thank FCT for their PhD grant (SFRH/BD/64911/2009) and Post-doc grant (SFRH/BPD/63746/2009), respectively.
Conflict of interest
We declare that we have no conflict of interest regarding this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soares, A.S., Costa, V.M., Diniz, C. et al. The combination of Cl-IB-MECA with paclitaxel: a new anti-metastatic therapeutic strategy for melanoma. Cancer Chemother Pharmacol 74, 847–860 (2014). https://doi.org/10.1007/s00280-014-2557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2557-y