Abstract
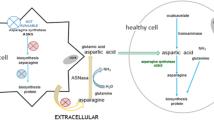
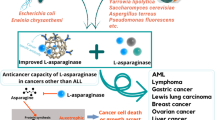
Asparaginases are among the most effective agents against acute lymphoblastic leukemia (ALL) and are Food and Drug Administration-approved for the treatment of pediatric and adult ALL. However, the efficacy of these drugs for the treatment of other hematologic malignancies particularly acute myeloid leukemia is not well established. The mechanism of action of asparaginases has thought to be related to a swift and sustained reduction in serum l-asparagine, which is required for rapid proliferation of metabolically demanding leukemic cells. However, asparagine depletion alone appears not to be sufficient for effective cytotoxic activity of asparaginase against leukemia cells, because glutamine can rescue asparagine-deprived cells by regeneration of asparagine via a transamidation chemical reaction. For this reason, glutamine reduction is also necessary for full anti-leukemic activity of asparaginase. Indeed, both Escherichia coli and Erwinia chrysanthemi asparaginases possess glutaminase enzymatic activity, and their administrations have shown to reduce serum glutamine level by deamidating glutamine to glutamate and ammonia. Emerging data have provided evidence that several types of neoplastic cells require glutamine for the synthesis of proteins, nucleic acids, and lipids. This fundamental role of glutamine and its metabolic pathways for growth and proliferation of individual malignant cells may identify a special group of patients whose solid or hematologic neoplasms may benefit significantly from interruption of glutamine metabolism. To this end, asparaginase products deserve a second look particularly in non-ALL malignant blood disorders. Here, we review mechanisms of anti-tumor activity of asparaginase focusing on importance of glutamine reduction, pharmacology of asparaginase products, in vitro activities as well as clinical experience of incorporating asparaginase in therapeutic regimens for non-ALL hematologic malignancies.

Similar content being viewed by others
References
Kidd JG (1953) Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. II. Studies on the nature of the active serum constituent: histological mechanism of the regression: tests for effects of guinea pig serum on lymphoma cells in vitro: discussion. J Exp Med 98(6):583–606
Broome JD (1961) Evidence that the L-asparaginase activity in guinea pig serum is responsible for antilymphoma effects. Nature 191:1114–1115
Yellin TO, Wriston JC Jr (1966) Antagonism of purified asparaginase from guinea pig serum toward lymphoma. Science 151(3713):998–999
Dolowy WC, Henson D, Cornet J, Sellin H (1966) Toxic and antineoplastic effects of L-asparaginase. Study of mice with lymphoma and normal monkeys and report on a child with leukemia. Cancer 19(12):1813–1819
Wade HE, Elsworth R, Herbert D, Keppie J, Sargeant K (1968) A new L-asparaginase with antitumour activity? Lancet 2(7571):776–777
Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui CH (2011) L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer 117(2):238–249
Dhavala P, Papageorgiou AC (2009) Structure of Helicobacter pylori L-asparaginase at 1.4 Å resolution. Acta Crystallogr D Biol Crystallogr 65(Pt 12):1253–1261
Soru E, Teodorescu M, Zaharia O, Szabados J, Rudescu K (1972) L-Asparaginase from the BCG strain of Mycobacterium bovis. I. Purification and in vitro immunosuppressive properties. Can J Biochem 50(11):1149–1157
DeJong PJ (1972) L-Asparaginase production by Streptomyces griseus. Appl Microbiol 23(6):1163–1164
Jones P, Kristiansen T, Einarsson M (1973) Purification and properties of L-asparaginase from Acinetobacter calcoaceticus. Biochem Biophys Acta 327:146
Lee B, Yang HJ, Henry GM, Seymour JP, Chibata I (1975) Crystallographic studies on L-asparaginase from Proteus vulgaris. II. Symmetry and location of the tetrameric molecule. J Biol Chem 250(16):6228–6231
Whelan HA, Wriston JC Jr (1974) Purification and properties of L-asparaginase from Serratia marcescens. Biochim Biophys Acta 365(1):212–222
Abuchowski A, Kafkewitz D, Davis FF (1979) A rapid purification procedure for L-asparaginase from Vibrio succinogenes. Prep Biochem 9(3):205–211
Kavitha A, Vijayalakshmi M (2012) A study on L-asparaginase of Nocardia levis MK-VL_113. Sci World J. 2012:160434
Lubkowski J, Palm GJ, Gilliland GL, Derst C, Rohm KH, Wlodawer A (1996) Crystal structure and amino acid sequence of Wolinella succinogenes L-asparaginase. Eur J Biochem 241(1):201–207
Lubkowski J, Wlodawer A, Ammon HL, Copeland TD, Swain AL (1994) Structural characterization of pseudomonas 7A glutaminase-asparaginase. Biochemistry 33(34):10257–10265
Ortlund E, Lacount MW, Lewinski K, Lebioda L (2000) Reactions of Pseudomonas 7A glutaminase-asparaginase with diazo analogues of glutamine and asparagine result in unexpected covalent inhibitions and suggests an unusual catalytic triad Thr-Tyr-Glu. Biochemistry 39(6):1199–1204
Amylon MD, Shuster J, Pullen J et al (1999) Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia 13(3):335–342
FDA Label for Asparaginase: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/101063s5169lbl.pdf
FDA Label for Erwinaze: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125359lbl.pdf
Abuchowski A, Kazo GM, Verhoest CR Jr et al (1984) Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys 7(2):175–186
Capizzi RL, Bertino JR, Skeel RT et al (1971) L-asparaginase: clinical, biochemical, pharmacological, and immunological studies. Ann Intern Med 74(6):893–901
Ho DH, Whitecar JP Jr, Luce JK, Frei E 3rd (1970) L-asparagine requirement and the effect of L-asparaginase on the normal and leukemic human bone marrow. Cancer Res 30(2):466–472
Kamisaki Y, Wada H, Yagura T, Matsushima A, Inada Y (1981) Reduction in immunogenicity and clearance rate of Escherichia coli L-asparaginase by modification with monomethoxypolyethylene glycol. J Pharmacol Exp Ther 216(2):410–414
Wada H, Imamura I, Sako M et al (1990) Antitumor enzyme: polyethylene glycol-modified asparaginase. Ann N Y Acad Sci 613:95–108
Patel S, Benfield P (1996) Pegasparaginase (polyethylene glycol L-asparaginase). Clin Immunother 5:492–496
Ettinger LJ, Kurtzberg J, Voute PA, Jurgens H, Halpern SL (1995) An open-label, multicenter study of polyethylene glycol-L-asparaginase for the treatment of acute lymphoblastic leukemia. Cancer 75(5):1176–1181
Cedar H, Schwartz JH (1967) Localization of the two asparaginases in anaerobically grown E. coli. J Biol Chem 247:3753–3755
Covini D, Tardito S, Bussolati O et al (2012) Expanding targets for a metabolic therapy of cancer: L-asparaginase. Recent Pat Anticancer Drug Discov 7(1):4–13
Schwartz JH, Reeves JY, Broome JD (1966) Two L-asparaginases from E. coli and their action against tumors. Proc Natl Acad Sci USA. 56(5):1516–1519
Cedar H, Schwartz JH (1968) Production of L-asparaginase II by Escherichia coli. J Bacteriol 96(6):2043–2048
Willis RC, Woolfolk CA (1974) Asparagine utilization in Escherichia coli. J Bacteriol 118(1):231–241
Casale TD, Sollitti P, Chesney RH (1983) Cytoplasmic L-asparaginase: isolation of a defective strain and mapping of ans A. J Bacteriol 154:513–515
Jennings MP, Beacham IR (1990) Analysis of the Escherichia coli gene encoding L-asparaginase II, ansB, and its regulation by cyclic AMP receptor and FNR proteins. J Bacteriol 172(3):1491–1498
Pui CH, Boyett JM, Relling MV et al (1999) Sex differences in prognosis for children with acute lymphoblastic leukemia. J Clin Oncol 17(3):818–824
Pui CH, Campana D, Evans WE (2001) Childhood acute lymphoblastic leukaemia—current status and future perspectives. Lancet Oncol 2(10):597–607
Kiriyama Y, Kubota M, Takimoto T et al (1989) Biochemical characterization of U937 cells resistant to L-asparaginase: the role of asparagine synthetase. Leukemia 3(4):294–297
Prager MD, Bachynsky N (1968) Asparagine synthetase in asparaginase resistant and susceptible mouse lymphomas. Biochem Biophys Res Commun 31(1):43–47
Avramis VI, Panosyan EH (2005) Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin Pharmacokinet 44(4):367–393
Panosyan EH, Grigoryan RS, Avramis IA et al (2004) Deamination of glutamine is a prerequisite for optimal asparagine deamination by asparaginases in vivo (CCG-1961). Anticancer research 24(2C):1121–1125
Souba WW (1993) Glutamine and cancer. Ann Surg 218(6):715–728
Cooney DA, Capizzi RL, Handschumacher RE (1970) Evaluation of l-asparagine metabolism in animals and man. Cancer Res 30(4):929–935
Mardashev SR, Nikolaev A, Kozlov EA, Petrii OP (1975) Purification of microbial asparaginases by the aid of affinity chromatography. Biokhimiia 40(1):78–82
Kamble VP, Rao RS, Borkar PS, Khobragade CN, Dawane BS (2006) Purification of L-asparaginase from a bacteria Erwinia carotovora and effect of a dihydropyrimidine derivative on some of its kinetic parameters. Indian J Biochem Biophys 43(6):391–394
Ho PP, Milikin EB, Bobbitt JL et al (1970) Crystalline L-asparaginase from Escherichia coli B. I. Purification and chemical characterization. J Biol Chem 245(14):3708–3715
Derst C, Henseling J, Rohm KH (2000) Engineering the substrate specificity of Escherichia coli asparaginase. II. Selective reduction of glutaminase activity by amino acid replacements at position 248. Protein Sci 9(10):2009–2017
Moola ZB, Scawen MD, Atkinson T, Nicholls DJ (1994) Erwinia chrysanthemi L-asparaginase: epitope mapping and production of antigenically modified enzymes. Biochem J 302(Pt 3):921–927
Kotzia GA, Labrou NE (2007) L-Asparaginase from Erwinia chrysanthemi 3937: cloning, expression and characterization. J Biotechnol 127(4):657–669
Avramis VI (2011) Asparaginases: a successful class of drugs against leukemias and lymphomas. J Pediatr Hematol Oncol 33(8):573–579
Jarrar M, Gaynon PS, Periclou AP et al (2006) Asparagine depletion after pegylated E. coli asparaginase treatment and induction outcome in children with acute lymphoblastic leukemia in first bone marrow relapse: a Children’s Oncology Group study (CCG-1941). Pediatr Blood Cancer 47(2):141–146
Avramis VI, Sencer S, Periclou AP et al (2002) A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood 99(6):1986–1994
Fine BM, Kaspers GJ, Ho M, Loonen AH, Boxer LM (2005) A genome-wide view of the in vitro response to L-asparaginase in acute lymphoblastic leukemia. Cancer Res 65(1):291–299
Shan J, Lopez MC, Baker HV, Kilberg MS (2010) Expression profiling after activation of the amino acid deprivation response in HepG2 human hepatoma cells. Physiol Genomics 41(3):315–327
Reinert RB, Oberle LM, Wek SA et al (2006) Role of glutamine depletion in directing tissue-specific nutrient stress responses to L-asparaginase. J Biol Chem 281(42):31222–31233
Willems L, Jacque N, Jacquel A et al (2013) Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 122(20):3521–3532
Offman MN, Krol M, Patel N et al (2011) Rational engineering of L-asparaginase reveals importance of dual activity for cancer cell toxicity. Blood 117(5):1614–1621
Ollenschlager G, Roth E, Linkesch W, Jansen S, Simmel A, Modder B (1988) Asparaginase-induced derangements of glutamine metabolism: the pathogenetic basis for some drug-related side-effects. Eur J Clin Invest 18(5):512–516
Panosyan EH, Seibel NL, Martin-Aragon S et al (2004) Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: children’s Cancer Group Study CCG-1961. J Pediatr Hematol Oncol 26(4):217–226
Douer D, Yampolsky H, Cohen LJ et al (2007) Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood 109(7):2744–2750
Pui CH (2009) Toward a total cure for acute lymphoblastic leukemia. J Clin Oncol 27(31):5121–5123
Schwartz MK, Lash ED, Oettgen HF, Tomato FA (1970) L-asparaginase activity in plasma and other biological fluids. Cancer 25(2):244–252
Dibenedetto SP, Di Cataldo A, Ragusa R, Meli C, Lo Nigro L (1995) Levels of l-asparagine in CSF after intramuscular administration of asparaginase from Erwinia in children with acute lymphoblastic leukemia. J Clin Oncol 13(2):339–344
Riccardi R, Holcenberg JS, Glaubiger DL, Wood JH, Poplack DG (1981) L-asparaginase pharmacokinetics and asparagine levels in cerebrospinal fluid of rhesus monkeys and humans. Cancer Res 41(11 Pt 1):4554–4558
Woo MH, Hak LJ, Storm MC et al (1999) Cerebrospinal fluid asparagine concentrations after Escherichia coli asparaginase in children with acute lymphoblastic leukemia. J Clin Oncol 17(5):1568–1573
Ho DH, Yap HY, Brown N et al (1981) Clinical pharmacology of intramuscularly administered L-asparaginase. J Clin Pharmacol 21(2):72–78
Haskell CM, Canellos GP, Leventhal BG et al (1969) L-asparaginase: therapeutic and toxic effects in patients with neoplastic disease. N Engl J Med 281(19):1028–1034
Kurtzberg J, Asselin B, Poplack D (1994) PEG L-asparaginase (PEG-ASP) pharmacology in pediatric patients with acute lymphoblastic leukemia (ALL). Proc Am Soc Clin Oncol 13:114
Leventhal BG, Henderson ES (1971) Therapy of acute leukemia with drug combinations which include asparaginase. Cancer 28(4):825–829
Capizzi RL (1993) Asparaginase revisited. Leuk Lymphoma 10(Suppl):147–150
Goldberg AI, Cooney DA, Glynn JP, Homan ER, Gaston MR, Milman HA (1973) The effects of immunization to L-asparaginase on antitumor and enzymatic activity. Cancer Res 33(2):256–261
Agrawal V, Woo J, Borthakur G, Kantarjian H, Frankel A (2013) Red blood cell-encapsulated L-asparaginase: potential therapy of patients with asparagine synthetase deficient acute myeloid leukemia. Protein Pept Lett 20(4):392–402
Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ (1993) Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol 11(9):1780–1786
Duval M, Suciu S, Ferster A et al (2002) Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group phase 3 trial. Blood 99(8):2734–2739
Moghrabi A, Levy DE, Asselin B et al (2007) Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109(3):896–904
Vieira Pinheiro JP, Ahlke E, Nowak-Gottl U et al (1999) Pharmacokinetic dose adjustment of Erwinia asparaginase in protocol II of the paediatric ALL/NHL-BFM treatment protocols. Br J Haematol 104(2):313–320
Boos J, Werber G, Ahlke E et al (1996) Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur J Cancer 32A(9):1544–1550
Clarkson B, Krakoff I, Burchenal J et al (1970) Clinical results of treatment with E. coli L-asparaginase in adults with leukemia, lymphoma, and solid tumors. Cancer 25(2):279–305
Crowther D (1971) L-asparaginase and human malignant disease. Nature 229(5281):168–171
Tardito S, Uggeri J, Bozzetti C et al (2007) The inhibition of glutamine synthetase sensitizes human sarcoma cells to L-asparaginase. Cancer Chemother Pharmacol 60(5):751–758
Lorenzi PL, Llamas J, Gunsior M et al (2008) Asparagine synthetase is a predictive biomarker of L-asparaginase activity in ovarian cancer cell lines. Mol Cancer Ther 7(10):3123–3128
Lorenzi PL, Reinhold WC, Rudelius M et al (2006) Asparagine synthetase as a causal, predictive biomarker for L-asparaginase activity in ovarian cancer cells. Mol Cancer Ther 5(11):2613–2623
Tardito S, Chiu M, Uggeri J et al (2011) L-Asparaginase and inhibitors of glutamine synthetase disclose glutamine addiction of beta-catenin-mutated human hepatocellular carcinoma cells. Curr Cancer Drug Targets 11(8):929–943
Zhang B, Dong LW, Tan YX et al (2013) Asparagine synthetase is an independent predictor of surgical survival and a potential therapeutic target in hepatocellular carcinoma. Br J Cancer 109(1):14–23
Scotti C, Sommi P, Pasquetto MV et al (2010) Cell-cycle inhibition by Helicobacter pylori L-asparaginase. PLoS ONE 5(11):e13892
Cappelletti D, Chiarelli LR, Pasquetto MV, Stivala S, Valentini G, Scotti C (2008) Helicobacter pylori L-asparaginase: a promising chemotherapeutic agent. Biochem Biophys Res Commun 377:1222–1226
Capizzi RL, Cheng Y (1981) Therapy of neoplasia with asparaginase. In: Holcenberg JS, Robert J (eds) Enzymes as drugs. Wiley, New York, pp 1–24
Winston J, Yellin TO (1973) L-asparaginase: a review. Adv Enzymol 39:185–248
Hill JM, Roberts J, Loeb E, Khan A, MacLellan A, Hill RW (1967) L-asparaginase therapy for leukemia and other malignant neoplasms. Remission in human leukemia. JAMA 202(9):882–888
Okada S, Hongo T, Yamada S et al (2003) In vitro efficacy of L-asparaginase in childhood acute myeloid leukemia. Br J Haematol 123(5):802–809
Zwaan CM, Kaspers GJ, Pieters R et al (2000) Cellular drug resistance profiles in childhood acute myeloid leukemia: differences between FAB types and comparison with acute lymphoblastic leukemia. Blood 96(8):2879–2886
Jun SA, Sepiashvili L, Kislinger T, Minden MD (2011) Investigating the potential use of L-asparaginase in myeloid leukemia. Blood (ASH Annual Meeting Abstracts) 118:3641
Takahashi H, Koh K, Kato M, Kishimoto H, Oguma E, Hanada R (2012) Acute myeloid leukemia with mediastinal myeloid sarcoma refractory to acute myeloid leukemia therapy but responsive to L-asparaginase. Int J Hematol 96(1):136–140
Narta UK, Kanwar SS, Azmi W (2007) Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol 61(3):208–221
Perel Y, Auvrignon A, Leblanc T et al (2002) Impact of addition of maintenance therapy to intensive induction and consolidation chemotherapy for childhood acute myeloblastic leukemia: results of a prospective randomized trial, LAME 89/91. Leucamie Aique Myeloide Enfant. J Clin Oncol 20(12):2774–2782
Wrobel G, Dobaczewski G, Kazanowska B et al (2000) Adverse reactions associated with the use of L-asparaginase during therapy of patients with nB non-Hodgkin’s lymphoma. Report of the Polish Paediatric Leukaemia/Lymphoma Study Group. Med Wieku Rozwoj 4(1 Suppl 2):67–72
Kobrinsky NL, Sposto R, Shah NR et al (2001) Outcomes of treatment of children and adolescents with recurrent non-Hodgkin’s lymphoma and Hodgkin’s disease with dexamethasone, etoposide, cisplatin, cytarabine, and l-asparaginase, maintenance chemotherapy, and transplantation: children’s Cancer Group Study CCG-5912. J Clin Oncol 19(9):2390–2396
Agrawal NR, Bukowski RM, Rybicki LA, Kurtzberg J, Cohen LJ, Hussein MA (2003) A phase I-II trial of polyethylene glycol-conjugated L-asparaginase in patients with multiple myeloma. Cancer 98(1):94–99
Ishida F, Kwong YL (2010) Diagnosis and management of natural killer-cell malignancies. Expert Rev Hematol 3(5):593–602
Jaccard A, Gachard N, Marin B et al (2011) Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117(6):1834–1839
Matsumoto Y, Nomura K, Kanda-Akano Y et al (2003) Successful treatment with Erwinia L-asparaginase for recurrent natural killer/T cell lymphoma. Leuk Lymphoma 44(5):879–882
Schwartz S, Morgenstern B, Capizzi RL (1982) Schedule dependent synergy and antagonism between high dose cytosine arabinoside and L-asparaginase in the L5178Y murine leukemia. Cancer Res 42:2191
Capizzi RL, Poole M, Cooper MR et al (1984) Treatment of poor risk acute leukemia with sequential high dose ARA-C and asparaginase. Blood 63(3):694–700
Capizzi RL, Davis R, Powell B et al (1988) Synergy between high-dose cytarabine and asparaginase in the treatment of adults with refractory and relapsed acute myelogenous leukemia- a cancer and Leukemia group B study. J Clin Oncol 6(3):499–508
Wells RJ, Woods WG, Lampkin BC et al (1993) Impact of high-dose cytarabine and asparaginase intensification on childhood acute myeloid leukemia: a report from the Children’s Cancer Group. J Clin Oncol 11(3):538–545
Horikoshi A, Takei K, Iriyama N et al (2009) Effect of L-asparaginase combined with vincristine and prednisolone on acute myeloblastic leukemia (M0) associated with non-Hodgkin Lymphoma. Acta Haematol 122:54–57
Fujita H, Iguchi M, Tachibana T et al (2006) Remission induction treatment for 6 patients of Jehovah’s witnesses with de novo acute leukemia. Blood (ASH Annual Meeting Abstracts). 108:4553
Buaboonnam J, Cao X, Pauley JL et al (2013) Sequential administration of methotrexate and asparaginase in relapsed or refractory pediatric acute myeloid leukemia. Pediatr Blood Cancer 60(7):1161–1164
Ahmed T, Holwerda S, Isom S, Lyerly S, Ellis LR, Manuel M, Dralle S, Dmitriy B, Klepin HD, Powell BL, Pardee T (2013) High dose cytarabine, mitoxantrone and L-asparaginase (HAMA) salvage for relapsed or refractory acute myeloid leukemia (AML) In the elderly. Blood (ASH Annual Meeting Abstracts). 122:2700
Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC (2013) Epidemiology of cancer-associated venous thrombosis. Blood 122(10):1712–1723
Howlader N, Noone AM, Krapcho M et al (2012) SEER Cancer Stat Rev, 1975–2010. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2010/. Based on November 2012 SEER data submission, posted to the SEER web site, April 2013. Accessed 19 Jan 2014
Emadi A, Jun SA, Tsukamoto T, Fathi AT, Minden MD, Dang CV (2013) Inhibition of glutaminase selectively suppresses the growth of primary AML cells with IDH mutations. Exp Hematol. doi:10.1016/j.exphem.2013.12.001
Conflict of interest
The authors report no relevant conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emadi, A., Zokaee, H. & Sausville, E.A. Asparaginase in the treatment of non-ALL hematologic malignancies. Cancer Chemother Pharmacol 73, 875–883 (2014). https://doi.org/10.1007/s00280-014-2402-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2402-3