Abstract
Purpose
The metabolism of pazopanib is primarily mediated by CYP3A4. The solubility of pazopanib is pH-dependent, and an elevated gastric pH may decrease its bioavailability. This study evaluated the effect of a potent CYP3A4 inhibitor, ketoconazole, and the proton pump inhibitor esomeprazole on the pharmacokinetics and safety of pazopanib and its metabolites.
Methods
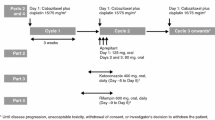
In Arm A, patients received pazopanib 400 mg alone once daily for 7 days followed by pazopanib 400 mg plus ketoconazole 400 mg once daily for 5 days. In Arm B, patients received pazopanib 800 mg once daily for 7 days, followed by pazopanib 800 mg plus esomeprazole 40 mg once daily for 5 days, and then pazopanib alone on the last day.
Results
Arm A enrolled 21 patients. In the presence of ketoconazole, mean area under the plasma concentration–time curve 24 h post-dose (AUC(0–24)) and mean maximum observed concentration (C max) of pazopanib increased by 66 and 45 %, respectively; mean AUC(0–24) and C max for pazopanib metabolites were lower or remained unchanged. Arm B enrolled 13 patients. In the presence of esomeprazole, mean pazopanib AUC(0–24) and C max decreased by 40 and 42 %, respectively; mean values of those parameters for metabolites of pazopanib also decreased.
Conclusions
Concomitant use of pazopanib with a strong CYP3A4 inhibitor should be avoided. If coadministration is necessary, pazopanib should be reduced to 400 mg. Concomitant use of pazopanib and proton pump inhibitors should also be avoided. Alternative dosing regimens that do not increase gastric pH at the time of pazopanib dosing should be considered.


Similar content being viewed by others
References
Harris PA, Boloor A, Cheung M et al (2008) Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-b enzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem 51:4632–4640
Sternberg CN, Davis ID, Mardiak J et al (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28:1061–1068
van der Graaf WT, Blay JY, Chawla SP et al (2012) Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379:1879–1886
Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, Hodge JP, Merkle EM, Pandite L (2009) Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 15:4220–4227
Goh BC, Reddy NJ, Dandamudi UB et al (2010) An evaluation of the drug interaction potential of pazopanib, an oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, using a modified Cooperstown 5 + 1 cocktail in patients with advanced solid tumors. Clin Pharmacol Ther 88:652–659
Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D (2011) Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects. Br J Clin Pharmacol 71:522–527
Dutreix C, Peng B, Mehring G, Hayes M, Capdeville R, Pokorny R, Seiberling M (2004) Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol 54:290–294
Johnson FM, Agrawal S, Burris H et al (2010) Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer 116:1582–1591
Di Gion P, Kanefendt F, Lindauer A, Scheffler M, Doroshyenko O, Fuhr U, Wolf J, Jaehde U (2011) Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on pyrimidines, pyridines and pyrroles. Clin Pharmacokinet 50:551–603
Miner P Jr, Katz PO, Chen Y, Sostek M (2003) Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol 98:2616–2620
Yin OQ, Gallagher N, Fischer D, Demirhan E, Zhou W, Golor G, Schran H (2010) Effect of the proton pump inhibitor esomeprazole on the oral absorption and pharmacokinetics of nilotinib. J Clin Pharmacol 50:960–967
Kapadia S, Hapani S, Wu S (2011) Risk of high-grade liver toxicity with pazopanib in patients with cancer: a meta-analysis. J Clin Oncol 29(suppl):abstract 4595
Olkkola KT, Backman JT, Neuvonen PJ (1994) Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther 55:481–485
Bjornsson TD, Callaghan JT, Einolf HJ et al (2003) The conduct of in vitro and in vivo drug–drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos 31:815–832
Heath EI, Chiorean EG, Sweeney CJ et al (2010) A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin Pharmacol Ther 88:818–823
Warrington S, Baisley K, Boyce M, Tejura B, Morocutti A, Miller N (2002) Effects of rabeprazole, 20 mg, or esomeprazole, 20 mg, on 24-h intragastric pH and serum gastrin in healthy subjects. Aliment Pharmacol Ther 16:1301–1307
Wilder-Smith C, Rohss K, Bokelund Singh S, Sagar M, Nagy P (2010) The effects of dose and timing of esomeprazole administration on 24-h, daytime and night-time acid inhibition in healthy volunteers. Aliment Pharmacol Ther 32:1249–1256
Acknowledgments
Financial support for this study and for medical editorial assistance was provided by Glaxo SmithKline Pharmaceuticals, Philadelphia, Pennsylvania. We thank William Sinkins, PhD, ProEd Communications, Inc., for his medical editorial assistance with this manuscript.
Conflict of interest
Authors Suttle and Tada are GlaxoSmithKline employees and stockholders. Author Botbyl’s company, Provonix, provides data services to GlaxoSmithKline. Author Tan has received research funding from GlaxoSmithKline. All other authors report no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical Trials Registration Number: NCT01205230.
Rights and permissions
About this article
Cite this article
Tan, A.R., Gibbon, D.G., Stein, M.N. et al. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol 71, 1635–1643 (2013). https://doi.org/10.1007/s00280-013-2164-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2164-3