Abstract
Purpose
Inhibition of DNA repair is emerging as a new therapeutic strategy for cancer treatment. One promising target is DNA-PK, a pivotal kinase in double-strand break repair. The purpose of this study was to further characterise the activity of the DNA-PK inhibitor NU7441, giving some new insights into the biology of DNA-PK.
Methods
We used NU7441, a potent DNA-PK inhibitor, to evaluate potential pharmacodynamic markers of DNA-PK inhibition, inhibition of DNA repair and chemo- and radio-potentiation in isogenic human cancer cells proficient (M059-Fus1) and deficient (M059 J) in DNA-PK.
Results
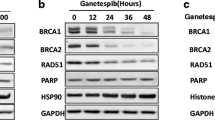
NU7441 strongly inhibited DNA-PK in cell lines (IC50 = 0.3 μM) but only weakly inhibited PI3 K (IC50 = 7 μM). The only available anti-phospho-DNA-PK antibody also recognised some phosphoprotein targets of ATM. NU7441 caused doxorubicin- and IR-induced DNA DSBs (measured by γ-H2AX foci) to persist and also slightly decreased homologous recombination activity, as assessed by Rad51 foci. Chemo- and radio-potentiation were induced by NU7441 in M059-Fus-1, but not in DNA-PK-deficient M059 J cells. DNA-PK was highly expressed in a chronic lymphocytic leukaemia sample but undetectable in resting normal human lymphocytes, although it could be induced by PHA-P treatment. In K652 cells, DNA-PK expression was not related to cell cycle phase.
Conclusion
These data confirm NU7441 not only as a potent chemo- and radio-sensitiser clinical candidate but also as a powerful tool to study the biology of DNA-PK.





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Lisby M, Rothstein R (2009) Choreography of recombination proteins during the DNA damage response. DNA Repair (Amst) 8(9):1068–1076
Uematsu N et al (2007) Autophosphorylation of DNA-PKCS regulates its dynamics at DNA double-strand breaks. J Cell Biol 177(2):219–229
Hartlerode AJ, Scully R (2009) Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J 423(2):157–168
Stavnezer J, Guikema JE, Schrader CE (2008) Mechanism and regulation of class switch recombination. Annu Rev Immunol 26:261–292
Bosma MJ, Carroll AM (1991) The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol 9:323–350
Smith GC, Jackson SP (1999) The DNA-dependent protein kinase. Genes Dev 13(8):916–934
Arlander SJ et al (2008) DNA protein kinase-dependent G2 checkpoint revealed following knockdown of ataxia-telangiectasia mutated in human mammary epithelial cells. Cancer Res 68(1):89–97
Muller C et al (1998) DNA-Dependent protein kinase activity correlates with clinical and in vitro sensitivity of chronic lymphocytic leukemia lymphocytes to nitrogen mustards. Blood 92(7):2213–2219
Willmore E et al (2008) DNA-dependent protein kinase is a therapeutic target and an indicator of poor prognosis in B-cell chronic lymphocytic leukemia. Clin Cancer Res 14(12):3984–3992
Leahy JJ et al (2004) Identification of a highly potent and selective DNA-dependent protein kinase (DNA-PK) inhibitor (NU7441) by screening of chromenone libraries. Bioorg Med Chem Lett 14(24):6083–6087
Zhao Y et al (2006) Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res 66(10):5354–5362
Shinichi Y, Koshimizu I, Yoshimi H, Matsuno T, Watanabe T, Tsuchida Y, Saitho K (2007) Treatment of prostate cancer, melanoma or hepatic cancer. Zenyaku Kogyo Kabushiki Kaisha, USA
Tsuchida R et al (2002) Detection of ATM gene mutation in human glioma cell line M059 J by a rapid frameshift/stop codon assay in yeast. Radiat Res 158(2):195–201
Rogakou EP et al (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273(10):5858–5868
Willmore E, de Caux S, Sunter NJ, Tilby MJ, Jackson GH, Austin CA, Durkacz BW (2004) A novel DNA-dependent protein kinase inhibitor, NU7026 potentiates the cytotoxicity of topoisomerase II poisons used in the treatment of leukemia. Blood 103(12):7
Zhao H, Piwnica-Worms H (2001) ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol 21:10
Bakkenist CJ, Kastan MB (2002) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:8
Datta J et al (2009) A new class of quinoline-based DNA hypomethylating agents reactivates tumor suppressor genes by blocking DNA methyltransferase 1 activity and inducing its degradation. Cancer Res 69(10):4277–4285
Terme JM et al (2009) TGF-beta induces degradation of TAL1/SCL by the ubiquitin-proteasome pathway through AKT-mediated phosphorylation. Blood 113(26):6695–6698
Hickson I et al (2004) Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res 64(24):9152–9159
Grimberg A (2003) Mechanisms by which IGF-I may promote cancer. Cancer Biol Ther 2(6):630–635
Johnson N et al (2009) Pre-clinical evaluation of cyclin-dependent kinase 2 and 1 inhibition in anti-estrogen-sensitive and resistant breast cancer cells. Br J Cancer 102(2):342–350
Lee JH, Paull TT (2007) Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene 26(56):7741–7748
McManus KJ, Hendzel MJ (2005) ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell 16(10):13
Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE (2006) H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci USA 103(26):6
Shrivastav M et al (2009) DNA-PKcs and ATM co-regulate DNA double-strand break repair. DNA Repair (Amst) 8(8):920–929
Mitchell J, Smith GC, Curtin NJ (2009) Poly(ADP-Ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int J Radiat Oncol Biol Phys 75(5):1520–1527
Hammel M et al (2010) Ku and DNA-dependent protein kinase (DNA-Pk) dynamic conformations and assembly regulate DNA binding and the initial nonhomologous end joining complex. J Biol Chem 285(2):1414–1423
Veuger SJ et al (2004) Effects of novel inhibitors of poly(ADP-ribose) polymerase-1 and the DNA-dependent protein kinase on enzyme activities and DNA repair. Oncogene 23(44):7322–7329
Zhuang HQ et al (2009) Radiosensitizing effects of gefitinib at different administration times in vitro. Cancer Sci 100(8):1520–1525
Acknowledgments
We gratefully acknowledge the financial support of CR UK (NJC, CC and DRN) and AstraZeneca (MT and JMM), Newcastle Cancer Centre-Medicinal Chemistry, Newcastle University, for providing NU7441 and ZSTK474 and Dr GCM Smith, AstraZeneca for providing KU55933.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tavecchio, M., Munck, J.M., Cano, C. et al. Further characterisation of the cellular activity of the DNA-PK inhibitor, NU7441, reveals potential cross-talk with homologous recombination. Cancer Chemother Pharmacol 69, 155–164 (2012). https://doi.org/10.1007/s00280-011-1662-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1662-4