Abstract
Objective
The aim of this study was to characterize the population pharmacokinetics of PM00104 (Zalypsis®) in cancer patients.
Methods
A total of 135 patients included in four phase I clinical trials who receive intravenous PM00104 at doses ranging from 53 to 5,000 μg/m2 and administered as 1-, 3-, or 24-h infusion every 3 weeks or as 1-h infusion on days 1, 8, and 15 of a 28-day cycle, or 1-h infusion daily during 5 consecutive days every 3 weeks were included in the analysis. Pharmacokinetic data were analyzed with non-linear mixed effect model using NONMEM VI software. The effect of selected patient covariates on PM00104 pharmacokinetics was investigated. Model evaluation was performed using predictive checks and non-parametric bootstrap.
Results
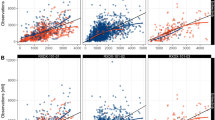
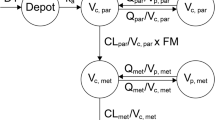
An open four-compartment catenary linear model with first-order elimination was developed to best describe the data. Plasma clearance and its between-subject variability was 43.7 L/h (34%). Volume of distribution at steady state was 822 L (117%). Within the range of covariates studied, age, sex, body size variables, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total bilirubin, lactate dehydrogenase, creatinine clearance, albumin, total protein, hemoglobin, performance status, liver metastases, dose-limiting toxicity, and stable disease for 3 months were not statistically related to PM00104 pharmacokinetic parameters. Bootstrap and posterior predictive check evidenced the model was deemed appropriate to describe the time course of PM00104 plasma concentrations in cancer patients.
Conclusions
The integration of phase I pharmacokinetic data demonstrated PM00104 linear elimination from plasma, dose proportionality up to 5,000 μg/m2, and time-independent pharmacokinetics. No clinically relevant covariates were identified as predictors of PM00104 pharmacokinetics.




Similar content being viewed by others
References
Leal JF, García-Hernández V, Moneo V, Domingo A, Bueren-Calabuig JA, Negri A, Gago F, Guillén-Navarro MJ, Avilés P, Cuevas C, García-Fernández LF, Galmarini CM (2009) Molecular pharmacology and antitumor activity of Zalypsis in several human cancer cell lines. Biochem Pharmacol 78:162–170
Guirouilh-Barbat J, Antony S, Pommier Y (2009) Zalypsis (PM00104) is a potent inducer of gamma-H2AX foci and reveals the importance of the C ring of trabectedin for transcription-coupled repair inhibition. Mol Cancer Ther 8:2007–2014
Ocio EM, Maiso P, Chen X, Garayoa M, Alvarez-Fernández S, San-Segundo L, Vilanova D, López-Corral L, Montero JC, Hernández-Iglesias T, de Alava E, Galmarini C, Avilés P, Cuevas C, San-Miguel JF, Pandiella A (2009) Zalypsis: a novel marine-derived compound with potent antimyeloma activity that reveals high sensitivity of malignant plasma cells to DNA double-strand breaks. Blood 113:3781–3791
Duan Z, Choy E, Jimeno JM, Cuevas Cdel M, Mankin HJ, Hornicek FJ (2009) Diverse cross-resistance phenotype to ET-743 and PM00104 in multi-drug resistant cell lines. Cancer Chemother Pharmacol 63:1121–1129
PM00104 (Zalypsis®) investigator’s brochure version 8.1. Pharma Mar 2008
Yin J, Aviles P, Lee W, Ly C, Guillen MJ, Munt S, Cuevas C, Faircloth G (2005) Development of a liquid chromatography/tandem mass spectrometry assay for the quantification of PM00104, a novel antineoplastic agent, in mouse, rat, dog, and human plasma. Rapid Commun Mass Spectrom 19:689–695
Yano Y, Beal SL, Sheiner LB (2001) Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28:171–192
Wang DD, Zhang S (2011) Standardized visual predictive check versus visual predictive check for model evaluation. J Clin Pharmacol. doi:10.1177/0091270010390040
Perez-Ruixo JJ, Zannikos P, Hirankarn S, Stuyckens K, Ludwig EA, Soto-Matos A, Lopez-Lazaro L, Owen JS (2007) Population pharmacokinetic meta-analysis of trabectedin (ET-743, Yondelis) in cancer patients. Clin Pharmacokinet 46:867–884
Nalda-Molina R, Valenzuela B, Ramon-Lopez A, Miguel-Lillo B, Soto-Matos A, Perez-Ruixo JJ (2009) Population pharmacokinetics meta-analysis of plitidepsin (Aplidin) in cancer subjects. Cancer Chemother Pharmacol 64:97–108
Acknowledgments
The authors thank Dr. Ricardo Nalda Molina for his comments and support at the beginning of this project. In addition, the authors would like to thank the patients, investigators, and their medical, nursing and laboratory staff who participated in the clinical trials included in the present study. In particular, we recognize the effort from the laboratory staff of ICON development solutions, which participated in the bioanalytical analysis of PM00104. Carlos Fernandez Teruel, Bernardo Miguel-Lillo, and Arturo Soto-Matos are employees of Pharma Mar SA, which supported this study. Consulting Projects for Research SL is consultant for Pharma Mar SA and received consultation fees for contributing to the current analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Ruixo, C., Valenzuela, B., Fernández Teruel, C. et al. Population pharmacokinetics of PM00104 (Zalypsis®) in cancer patients. Cancer Chemother Pharmacol 69, 15–24 (2012). https://doi.org/10.1007/s00280-011-1644-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1644-6