Abstract
Background
Antitubulin agents exhibit antiangiogenic effects in vitro and in vivo. We evaluated the safety and feasibility of administering a metronomic schedule of oral vinorelbine designed to optimize its antiangiogenic effects.
Methods
Patient with advanced cancer who had progressive disease after standard therapy received oral vinorelbine 3 times weekly (i.e., Monday, Wednesday, Friday) at the 6 dose levels ranging from 20 mg (1 week on, 1 week off) to 50 mg (3 weeks on, 1 week off) in cohorts of 3–6 patients at each dose level using a standard phase I design. Dose-limiting toxicity (DLT) during cycle 1 included: (1) neutrophil nadir < 500/μL attributed to therapy, (2) platelet nadir < 50,000/μL attributed to therapy, (3) grade 3–4 non-hematologic toxicity attributed to therapy, and (4) neutropenia associated with grade 2 fever (i.e., febrile neutropenia).
Results
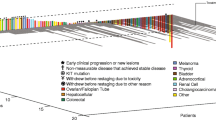
Nineteen patients received 50 cycles of therapy (range 1–11 cycles) at 6 dose levels. There were no dose-limiting toxic events. There were no consistent changes in serum TIE-2 or VCAM-1 levels, or urinary VEGF. One patient with renal cell carcinoma had stable disease for 9 months, and another patient with metastatic prostate cancer had a 70% decline in serum prostate-specific antigen, which lasted 4 months.
Conclusions
Oral vinorelbine may be given using a metronomic schedule, 50 mg thrice weekly for three of 4 weeks, with minimal toxicity in patients with advanced cancer.

Similar content being viewed by others
References
Binet S, Chaineau E, Fellous A et al (1990) Immunofluorescence study of the action of navelbine, vincristine and vinblastine on mitotic and axonal microtubules. Int J Cancer 46:262–266
Binet S, Fellous A, Lataste H et al (1989) In situ analysis of the action of Navelbine on various types of microtubules using immunofluorescence. Semin Oncol 16:5–8
Fellous A, Ohayon R, Vacassin T et al (1989) Biochemical effects of Navelbine on tubulin and associated proteins. Semin Oncol 16:9–14
Cvitkovic E, Izzo J (1992) The current and future place of vinorelbine in cancer therapy. Drugs 44(Suppl 4):36–45 (discussion 66–9)
Nasti G, Errante D, Talamini R et al (2000) Vinorelbine is an effective and safe drug for AIDS-related Kaposi’s sarcoma: results of a phase II study. J Clin Oncol 18:1550–1557
Rule S, Tighe M, Davies S et al (1998) Vinorelbine in the treatment of lymphoma. Hematol Oncol 16:101–105
Tirelli U, Balzarotti M, Uziel L et al (2006) Vinorelbine and prednisone in frail elderly patients with intermediate-high grade non-Hodgkin’s lymphomas. Ann Oncol 17:533
Belotti D, Vergani V, Drudis T et al (1996) The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res 2:1843–1849
Hotchkiss KA, Ashton AW, Mahmood R et al (2002) Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (Taxotere): association with impaired repositioning of the microtubule organizing center. Mol Cancer Ther 1:1191–1200
Vacca A, Iurlaro M, Ribatti D et al (1999) Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood 94:4143–4155
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4:423–436
Hanahan D, Bergers G, Bergsland E (2000) Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 105:1045–1047
Seidman AD, Berry D, Cirrincione C et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642–1649
Sparano JA, Wang M, Martino S et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Belani CP, Ramalingam S, Perry MC et al (2008) Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol 26:468–473
Rivera E, Mejia JA, Arun BK et al (2008) Phase 3 study comparing the use of docetaxel on an every-3-week versus weekly schedule in the treatment of metastatic breast cancer. Cancer 112:1455–1461
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Schuette W, Nagel S, Blankenburg T et al (2005) Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol 23:8389–8395
Rowinsky EK, Noe DA, Trump DL et al (1994) Pharmacokinetic, bioavailability, and feasibility study of oral vinorelbine in patients with solid tumors. J Clin Oncol 12:1754–1763
Depierre A, Freyer G, Jassem J et al (2001) Oral vinorelbine: feasibility and safety profile. Ann Oncol 12:1677–1681
Alexiou D, Karayiannakis AJ, Syrigos KN et al (2001) Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. Eur J Cancer 37:2392–2397
Caine GJ, Blann AD, Stonelake PS et al (2003) Plasma angiopoietin-1, angiopoietin-2 and Tie-2 in breast and prostate cancer: a comparison with VEGF and Flt-1. Eur J Clin Invest 33:883–890
Bocci G, Man S, Green SK et al (2004) Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res 64:6616–6625
Eisenhauer EA, O’Dwyer PJ, Christian M et al (2000) Phase I clinical trial design in cancer drug development. J Clin Oncol 18:684–692
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of Canada. J Natl Cancer Inst 92:205–216
Briasoulis E, Pappas P, Puozzo C et al (2009) Dose-ranging study of metronomic oral vinorelbine in patients with advanced refractory cancer. Clin Cancer Res 15:6454–6461
Wong AL, Haroon ZA, Werner S et al (1997) Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res 81:567–574
Sato TN, Tozawa Y, Deutsch U et al (1995) Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376:70–74
Eisen T, Boshoff C, Mak I et al (2000) Continuous low dose Thalidomide: a phase II study in advanced melanoma, renal cell, ovarian and breast cancer. Br J Cancer 82:812–817
Rice GE, Bevilacqua MP (1989) An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science 246:1303–1306
Acknowledgments
This study was supported in part by Department of Health and Human Services grant P30-133330.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajdev, L., Negassa, A., Dai, Q. et al. Phase I trial of metronomic oral vinorelbine in patients with advanced cancer. Cancer Chemother Pharmacol 68, 1119–1124 (2011). https://doi.org/10.1007/s00280-011-1580-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1580-5