Abstract
Purpose
Approximately 20–30% of breast cancer tumors overexpress or amplify human epidermal growth factor receptor 2 (HER2). The role of this receptor in the progression of HER2+ breast cancer and resistance to certain anticancer monotherapies was investigated. The results of several pre-clinical and clinical trials, with the aim of determining the most safe and effective course of treatment for HER2+ breast cancer, were also thoroughly examined.
Methods
A thorough search of databases including Pubmed, Springer, and The American Society of Clinical Oncology was performed, and pertinent studies were identified. The most relevant studies were preclinical, phase II, and III clinical trials identifying the function of the HER2 receptor in HER2+ breast cancer progression, as well as studies assessing the efficacy of monotherapy and combination therapy in the treatment of this aggressive form of cancer.
Results
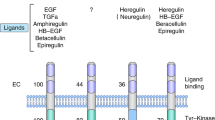
The HER2 receptor belongs to a family of receptors that consists of four cell-surface receptors (HER1-4) that share strong homology with the epidermal growth factor receptor (EGFR). All HER receptors interact with specific types of ligands to induce receptor activation, except for HER2, for which no known ligand has yet been identified. HER2 is activated by forming dimers with other HER receptors, and this results in a stronger and more prolonged signal transduction event. When expressed at normal levels, HER2 regulates cell growth, differentiation, and survival. However, under pathological conditions of HER2 overexpression, numerous HER2 heterodimers are formed resulting in aggressive tumor growth. Therefore, the prognosis associated with HER2-positive breast cancer is usually poor. A specific cohort of patients with breast cancer whose tumors test both hormone receptor (estrogen receptor [ER] and progesterone receptor [PR]) and HER2 positive have been found to be resistant to targeted hormone therapy. Studies investigating the etiology of this resistance have found that the cell membrane estrogen receptor communicates with HER2 in promoting the release of ER coactivators that cause the endocrine drug and selective estrogen receptor modulator, tamoxifen, to act as an agonist rather than an antagonist of the hormone estrogen. Thus, research has directed its inquiry toward the development of therapies specifically targeting HER2. The development of trastuzumab, a recombinant monoclonal antibody against HER2, initially proved to be a well-tolerated first line of treatment. However, in the long-term patients, trastuzumab was shown to develop resistance to this monotherapy. Therefore, research on HER2 positive breast cancer has focused on the study of different anti-HER2 combination therapies over the past decade.
Conclusions
While the development and approval of the HER2-targeted recombinant monoclonal antibody trastuzumab (Herceptin) has been efficacious in slowing HER2 cancer progression, combining this and other anti-HER2 therapy with either chemotherapy or endocrine therapy has proven more effective in improving overall and progression free survival.

Similar content being viewed by others
References
Jemal A, Siegel R, Ward E et al (2008) Cancer statistics, 2008. Cancer J Clin 58:71–96
Prat A, Baselga J (2008) The role of hormone therapy in the management of the hormonal-receptor-positive breast cancer with co-expression of HER2. Nat Clin Pract Oncol 5(9):531–542
Bartlett JMS, Going JJ, Mallon EA et al (2001) Evaluating HER2 amplification and expression in breast cancer. J Pathol 195:422–428
Lipton A, Ali SM, Leitzel K et al (2002) Elevated Serum HER-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol 20(6):1467–1472
Chang JC (2007) HER2 inhibition: from discovery to clinical practice. Clin Cancer Res 13:1–3
Rubin I, Yarden Y (2001) The basic biology of HER2. Ann Oncol 12(Suppl. 1):S3–S8
Cooke T (2000) What is HER2? Eur J Oncol Nurs 4(Suppl. 1):2–9
Ross JS, Fletcher JA, Linette GP et al (2003) The HER-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncol 8:307–325
Liu Y, El-Ashry D, Chen D et al (1995) MCF-7 breast cancer cells overexpressing transfected c-erbB-2 have an in vitro growth advantage in estrogen-depleted conditions and reduced estrogen-dependence and tamoxifen-sensitivity in vivo. Breast Cancer Res Treat 34:97–117
Kurokawa H, Lenferink AEG, Simpson JF et al (2000) Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res 60:5887–5894
Osborne KC, Bardou V, Hopp TA (2003) Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95(5):353–361
Shou J, Massarweh S, Osborne KC et al (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96(12):926–935
Osborne KC (1998) Tamoxifen in the treatment of breast cancer. N Engl J Med 339(22):1609–1618
Schiff R, Massarweh SA, Shou J et al (2004) Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 10:331s–336s
Smith CL, Nawaz Z, O’Malley BW (1997) Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11(6):657–666
Razandi M, Pedram A, Park ST, Levin ER (2003) Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 278(4):2701–2712
Font De Mora J, Brown M (2000) A1B1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol 20(14):5041–5047
Hendriks BS, Orr G, Wells A, Wiley HS, Lauffenburger DA (2005) Parsing ERK activation reveal quantitatively equivalent contributions from EGFR and HER2 in human mammary epithelial cells. J Biol Chem 280:6157–6169
Cobleigh MA, Vogel CL, Tripathy D et al (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648
Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Vogel CL, Cobleigh MA, Tripathy D et al (2002) Efficacy and Safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726
Vogel C, Cobleigh MA, Tripathy D et al (2001) First-line, single-agent Herceptin (trastuzumab) in metastatic breast cancer: a preliminary report. Eur J Cancer 37:S25–S29
Vogel CL, Cobleigh MA, Tripathy D et al (2001) First-line herceptin monotherapy in metastatic breast cancer. Oncology 61(Suppl. 2):37–42
Suter TM, Cook-Bruns N, Barton C (2004) Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast 13:173–183
Burstein HJ, Storniolo AM, Franco S et al (2008) A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol 19:1068–1074
Nahta R, Yuan LXH, Du Y, Esteva FJ (2007) Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther 6(2):667–674
Baselga J, Albanell J, Ruiz A et al (2005) Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol 23:5323–5333
Gomez HL, Doval DC, Chavez MA et al (2008) Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 26(18):2999–3005
Avigan D, Vasir B, Gong J et al (2004) Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res 10:4699–4708
Disis ML, Gooley TA, Rinn K et al (2002) Generation of T-Cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 20(11):2624–2632
Disis ML, Schiffman K, Guthrie K et al (2004) Effect of dose on immune response in patients vaccinated with an HER-2/neu intracellular domain protein-based vaccine. J Clin Oncol 22(10):1916–1925
Jakesz R, Jonat W, Gnant M et al (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ANCSG trial 8 and ARNO 95 trial. Lancet 366:455–462
Ellis MJ, Coop A, Singh B et al (2001) Letrozole is more effective Neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or Erb-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 19(18):3808–3816
Mouridsen H, Gershanovich M, Sun Y et al (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the international letrozole breast cancer group. J Clin Oncol 19(10):2596–2606
Kaufmann M, Jonat W, Hilfrich J et al (2007) Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 study. J Clin Oncol 25(19):2664–2669
Muss HB, Thor AD, Berry DA et al (1994) c-erbB-2 Expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med 330(18):1260–1266
Paik S, Bryant J, Tan-Chiu E et al (2000) HER2 and choice of adjuvant chemotherapy for invasive breast cancer: national surgical adjuvant breast and bowel project protocol B-15. J Natl Cancer Inst 92(24):1991–1998
Petit T, Wilt M, Velten M et al (2004) Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Eur J Cancer 40:205–211
Pritchard KI, Shepherd LE, O’Malley FP et al (2006) HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 354(20):2103–2111
Tanner M, Isola J, Wiklund T et al (2006) Topoisomerase IIα gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in HER-2/neu-amplified breast cancer: Scandinavian breast group trial 9401. J Clin Oncol 24(16):2428–2436
Levine MN, Bramhall VH, Pritchard KI et al (1998) A randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methrotrexate, and fluorouracil in premenopausal women with node positive breast cancer. J Clin Oncol 16:2651–2658
Levine MN, Pritchard KI, Bramhall VH et al (2005) A randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methrotrexate, and fluorouracil in premenopausal women with node positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol 23:5166–5170
Gennari A, Sormani MP, Pronzato P et al (2008) HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst 100(1):14–20
Limer JL, Speirs V (2004) Phytoestrogens and breast cancer chemoprevention. Breast Cancer Res 6(3):119–127
Lu L-J, Anderson KE, Grady JJ, Kohen F, Nagamani M (2000) Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer Res 60:4112–4121
Kumar NB, Cantor A, Allen K, Riccardi D, Cox CE (2002) The specific role of isoflavones on estrogen metabolism in premenopausal women. Cancer 94:1166–1174
Grube BJ, Eng ET, Kao Y-C, Kwon A, Chen S (2001) White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J Nutr 131:3288–3293
Lu L-J W, Cree M, Josyula S, Nagamani M, Grady JJ, Anderson KE (2000) Increased urinary excretion of 2-hydroxyestrone but not 16α-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res 60:1299–1305
Sakla MS, Shenouda NS, Ansell PJ, MacDonald RS, Lubahn DD (2007) Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocrine 32:69–78
Provinciali M, Re F, Donnini A et al (2005) Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer 115:36–45
Baselga J (2001) Herceptin Alone or in Combination with Chemotherapy in the Treatment of HER2-Positive Metastatic Breast Cancer: Pivotal Trials. Oncology 61(Suppl. 2):14–21
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metatstatic breast cancer that overexpresses HER2. N Engl J Med 334(11):783–792
Buzdar AU, Ibrahim NK, Francis D et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23:3676–3685
Hurley J, Doliny P, Reis I et al (2006) Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol 24(12):1831–1838
Pegram MD, Pienkowski T, Northfelt DW et al (2004) Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst 96(10):759–769
Nahta R, Esteva FJ (2006) Molecular mechanisms of trastuzumab resistance. Breast Cancer Res 8(6):667–674
Cameron D, Casey M, Press M et al (2008) A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 112:533–543
Marcom PK, Isaacs C, Harris L et al (2007) The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat 102:43–49
Nahta R, Hung M-C, Esteva FJ (2004) The HER2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 64:2343–2346
Swaby R, Blackwell K, Jiang Z et al (2009) Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: a phase I/II study. J Clin Oncol 27(15S):1004
Lewis Philips GD, Li G, Dugger DL (2008) Targeting HER2 positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 68:9280
Kamal A, Thao L, Sensintaffer J et al (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407–410
Yu K, Toral-Barza L, Discafani C et al (2001) mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer 8:249–258
Moy B, Goss PE (2006) Lapatinib: current status and future directions in breast cancer. Oncol 11(10):1047–1057
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davoli, A., Hocevar, B.A. & Brown, T.L. Progression and treatment of HER2-positive breast cancer. Cancer Chemother Pharmacol 65, 611–623 (2010). https://doi.org/10.1007/s00280-009-1208-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1208-1