Abstract
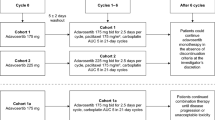
Larotaxel (XRP9881, RPR109881), a novel semi-synthetic taxoid that shares a mode of action with the taxanes docetaxel and paclitaxel, was active in preclinical studies against a broad spectrum of tumour cells and tumour models refractory/resistant to taxanes, and have demonstrated clinical activity in taxane pre-treated/resistant metastatic breast cancer (MBC) patients. The current phase I dose-escalation study sought to define in Japanese patients with advanced solid tumours the maximum tolerated dose (MTD) and recommended dose (RD) of larotaxel administered as a 1-h intravenous infusion every 3 weeks. Eighteen patients were treated in total with 11 of those (61%) having previously received a docetaxel- or paclitaxel-based regimen. The MTD was defined as 90 mg/m2 following the occurrence of dose-limiting toxicities (DLTs) in two of five patients treated at this dose level including grade 4 neutropenia lasting >7 days or febrile neutropenia. The RD for phase II was consequently 75 mg/m2 q3w, with no DLTs in the six patients treated. The principal toxicity and DLT was neutropenia, with or without neutropenic complications. Partial responses were reported for 2 of 18 (11%) treated patients and a further 8 achieved stable disease (44%). The clearance 19.1–31.9 L/h was similar to that observed in Caucasian subjects with value of 33.0 ± 10.7 L/h. In conclusion, larotaxel 75 mg/m2, administered as a 1-h infusion every 3 weeks, appeared to be clinically tolerable in this Japanese patient population and showed early indications of activity.

Similar content being viewed by others
References
Yamaguchi K (2002) Overview of cancer control programs in Japan. Jpn J Clin Oncol 32(Suppl):S22–S31
Gligorov J, Lotz JP (2004) Preclinical pharmacology of the taxanes: implications of the differences. Oncologist 9(Suppl 2):3–8
Yvon AM, Wadsworth P, Jordan MA (1999) Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell 10:947–959
Fabbri F, Carloni S, Brigliadori G et al (2006) Sequential events of apoptosis involving docetaxel, a microtubule-interfering agent: a cytometric study. BMC Cell Biol 7:6
Woods CM, Zhu J, McQueney PA et al (1995) Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med 1:506–526
Fojo T, Menefee M (2007) Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol 18(Suppl 5):v3–v8
Bissery MC (2001) Preclinical evaluation of new taxoids. Curr Pharm Des 7:1251–1257
Engels FK, Sparreboom A, Mathot RA et al (2005) Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. Br J Cancer 93:173–177
Bissery M-C, Vrignaud P, Combeau C et al (2004) Preclinical evaluation of XRP9881A, a new taxoid. In: Proceedings of the 95th annual meeting of the American association for cancer research, p 45 (abstr 5430)
Barthier S, Dieras V, Kalla S et al (1998) A phase I and pharmacokinetics (PK) study of RPR 109881A given as 6-h iv infusion in patients (pts) with advanced solid tumors. Proc Am Soc Clin Oncol 16:(abstr 747)
Gelmon KA, Latreille J, Tolcher A et al (2000) Phase I dose-finding study of a new taxane, RPR 109881A, administered as a 1-h intravenous infusion days 1 and 8 to patients with advanced solid tumors. J Clin Oncol 18:4098–4108
Kurata T, Shimada Y, Tamura T et al (2000) Phase I and pharmacokinetic study of a new taxoid, RPR 109881A, given as a 1-h intravenous infusion in patients with advanced solid tumors. J Clin Oncol 18:3164–3171
Sessa C, Cuvier C, Caldiera S et al (2002) Phase I clinical and pharmacokinetic studies of the taxoid derivative RPR 109881A administered as a 1-h or a 3-h infusion in patients with advanced solid tumors. Ann Oncol 13:1140–1150
Slaughter M, Pazdur R, Hoff PM et al (1998) Phase I trial of RPR 109881A (RPR), a novel taxoid derivative, administered as a 24-h continuous infusion. Proc Am Soc Clin Oncol 16:(abstr 748)
Vernillet L, Semiond D, Vergniol JC et al (1998) Pharmacokinetics of a new taxoid (RPR 109881A): comparison of five different administration schedules. Proc Am Soc Clin Oncol 16:(abstr 749)
Dieras VC, Limantani SA, Lortholary A et al (2003) A multicentre, non randomized phase II study with RPR 109881A in metastatic breast cancer (MBC) patients (pts). Proc Am Soc Clin Oncol 22:(abstr 565)
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Smith TJ, Khatcheressian J, Lyman GH et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
Benson ABIII, Ajani JA, Catalano RB et al (2004) Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol 22:2918–2926
Mielke S, Sparreboom A, Mross K (2006) Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer 42:24–30
Acknowledgments
We would like to thank the patients and their families and the medical staff at both clinical centres.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, N., Boku, N. & Minami, H. Phase I study of larotaxel administered as a 1-h intravenous infusion every 3 weeks to Japanese patients with advanced solid tumours. Cancer Chemother Pharmacol 65, 129–136 (2009). https://doi.org/10.1007/s00280-009-1014-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1014-9