Abstract
Purpose
The body distribution of total radioactivity (TR) and bortezomib was investigated in male Sprague-Dawley rats after single and repeated i.v. (bolus) administration with 14C-labelled bortezomib (VELCADE®) (0.2 mg/kg; 0.28 MBq./kg).
Methods
Bortezomib was dosed on days 1, 4, 8, and 11 (i.e. a clinical dosing cycle) and the animals were sacrificed at selected time points following single and repeated dose administration for the quantification of TR in blood, plasma, and various tissues by liquid scintillation counting following organ dissection or by quantitative whole body autoradiography. In selected tissues, bortezomib levels were determined by LC-MS/MS.
Results
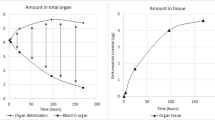
In general, plasma TR levels were less than 10% of the corresponding blood concentrations. TR was rapidly and widely distributed to the tissues with only limited penetration into the central nervous system (CNS). In the tissues, highest levels of TR were measured in bortezomib-eliminating organs (liver and kidney), lymphoid tissues, and regions of rapidly dividing cells (e.g. the bone marrow, intestinal mucosa). Low TR concentrations were found in the CNS (tissue-to-blood ratio of ∼0.05 after repeated dosing). With the exception of the liver, TR consisted almost exclusively of the parent drug. Tissue concentrations of TR and bortezomib increased up to about threefold from the first to the third dose administration, after which they remained constant.
Conclusion
No undue tissue accumulation of TR and of bortezomib was observed in rats following a full clinical dosing cycle of bortezomib.




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the concentration–time curve
- C max :
-
Maximum concentration
- CNS:
-
Central nervous system
- UD:
-
Unchanged drug
- LC-MS/MS:
-
Liquid chromatography coupled with tandem mass spectrometry
- LSC:
-
Liquid scintillation counting
- LLOQ:
-
Lower limit of quantification
- QC:
-
Quality control
- QWBA:
-
Quantitative whole body autoradiography
- T/B:
-
Tissue-to-blood ratio
- TR:
-
Total radioactivity
References
Montagut C, Rovira A, Mellado B, Gascon P, Ross JS, Albanell J (2005) Preclinical and clinical development of the proteasome inhibitor bortezomib in cancer treatment. Drugs Today 41(5):299–315
Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, Bradley S, Gobburu JV, Goheer A, Lee S-L, Leighton J, Liang CY, Lostritto RT, Guinn WD, Morse DE, Rahman A, Rosario LA, Verbois SL, Williams G, Wang Y-C, Pazdur R (2004) Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res 10:3954–3964
Ludwig H, Khayat H, Giaccone G, Facon T (2005) Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer 104:1794–1807
Richardson PG, Mitsiades C, Hideshima T, Anderson KC (2006) Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med 57:33–47
Voorhees PM, Orlowski RZ (2006) The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol 46:189–213
Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ (1999) Proteasome inhibitors: a novel class of effective antitumor agents. Cancer Res 59:2615–2622
ICH, Committee for Medicinal Products for Human Use (CPMP) (November 1994) S3B: pharmacokinetics: guidance for repeated dose tissue distribution studies (CPMP/ICH/385/95)
McNaught KS, Perl DP, Brownell AL, Olanow CW (2004) Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol 56(1):149–162
Ardley HC, Hung CC, Robinson PA (2005) The aggravating role of the ubiquitin-proteasome system in neurodegeneration. FEBS Lett 579:571–576
Upadhya SC, Hegde AN (2005) Ubiquitin-proteasome pathway components as therapeutic maladies. Curr Pharm Des 11:3807–3828
FDA, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM) (May 2001). Bioanalytical Method Validation
Daniels J, LaButti J, Chavan A, Huang R, Parsons I, Gan L, Miwa G (2003) Mechanistic investigations into the protein and glutathion-mediated deboronation of the peptide boronic acid proteasome inhibitors bortezomib (VELCADE™). Drug Metab Rev 35:44
Pekol T, Daniels JS, Labutti J, Parsons I, Nix D, Baronas E, Hsieh F, Gan LS, Miwa G (2005) Human metabolism of the proteasome inhibitor bortezomib: identification of circulating metabolites. Drug Metab Dispos 33:771–777
Uttamsingh V, Lu C, LaButti J, Daniels J, Huang R, Gan L, Miwa G (2003) Relative contribution of CYP isoforms to the liver microsomal intrinsic clearance of bortezomib (VELCADE™). Drug Metab Rev 35:184
Uttamsingh V, Lu C, Miwa G, Gan LS (2005) Relative contributions of the five major human cytochrome P450, 1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the proteasome inhibitor bortezomib. Drug Metab Dispos 33:1723–1728
Miyakoshi S, Kami M, Yuji K, Matsumura T, Takatoku M, Sasaki M, Narimatsu H, Fujii T, Kawabata M, Taniguchi S, Ozawa K, Oshimi K (2006) Severe pulmonary complications in Japanese patients after bortezomib treatment for refractory multiple myeloma. Blood 107(9):3492–3494
Yu LJ, Riordan B, Hatsis P, Brockman A, Daniels S, Stagliano N, Finklestein S, Ren J, Milton M, Miwa G (2006) Study of brain and whole blood PK/PD of bortezomib (VELCADE®) in rat models. J Clin Oncol. ASCO annual meeting proceedings part I, vol 24, no. 18S (June 20 Supplement):12036
Acknowledgments
The authors would also like to express their gratitude to Willem Meuldermans (J&JPRD), Gerald Miwa, and Li Yu (Millennium Pharmaceuticals) for reviewing the manuscript. Larry Weaner and David Hoerr (J&JPRD) are acknowledged for synthesizing 14C-bortezomib. Helena Geys is thanked for her contribution to the data analysis and Mia Vanthienen is acknowledged for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hemeryck, A., Geerts, R., Monbaliu, J. et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14C-bortezomib. Cancer Chemother Pharmacol 60, 777–787 (2007). https://doi.org/10.1007/s00280-007-0424-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0424-9