Abstract
Purpose
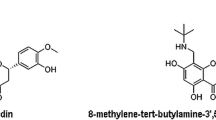
To compare the pharmacokinetics and tissue distribution (both normal and tumor) of cryptophycin 52 (C-52) and its putative chlorohydrin prodrug cryptophycin 55 (C-55) in a murine model and to investigate a possible mechanism behind the superior activity of C-55.
Methods
Mammary adenocarcinoma 16/c tumor-bearing mice were treated with an i.v. bolus of 11 mg/kg C-52 or 38 mg/kg C-55 in Cremophor-alcohol. At predetermined time intervals, C-52 and C-55 concentrations in plasma, liver, kidney, small intestine and tumors were measured using a previously described HPLC method. Pharmacokinetic parameters were computed using noncompartmental methods. Tissue (both normal and tumor) to plasma ratios as a function of time were also calculated for comparison.
Results
Both C-52 and C-55 were rapidly distributed into different tissues including tumors following i.v. administration. However, the affinities of these compounds towards different tissues were different. Thus, the half-lives (minutes) of C-55 were in the decreasing order liver (725), intestine (494), tumor (206), kidney (62) and plasma (44), whereas the AUC values (μg·min/ml) were in the order tumor (9077), liver (7734), kidney (6790), plasma (2372) and intestine (2234). For C-52, the half-lives (minutes) were in the decreasing order liver (1333), kidney (718), intestine (389), tumor (181) and plasma (35), and the AUC values (μg·min/ml) were in the order kidney (1164), liver (609), intestine (487), plasma (457) and tumor (442). The relative exposures to C-52 after i.v. injection of C-55 were plasma 3.9%, tumor 80.8%, kidney 3.4%, liver 1.1% and intestine 2.8%. Although plasma exposure to C-52 following C-55 administration was relatively small, the use of C-55 to deliver C-52 increased the retention of C-52 and its AUC in tumor compared to direct injection of C-52. Simultaneously, this approach shortened C-52 retention in all normal tissues studied.
Conclusions
The distribution of C-55 and its bioconversion to C-52 in different organs and tumor tissue observed in this study suggest the ability of C-55 to target tumor tissue, creating a depot of C-52 in tumor. Increased C-52 exposure of tumor, with concomitant decreased exposure of normal tissue, is a contributing factor to the superior activity of C-55 versus C-52. However, except in the case of tumor tissue in which 81% of C-55 converts to C-52, only a minor amount of C-55 may serve as a prodrug for C-52, whereas the majority is handled by the biosystem through a different route of elimination. Tissue distribution combined with rate of conversion may be an important determinant of the relative effectiveness of other epoxide-chlorohydrin pairs of cryptophycins.




Similar content being viewed by others
References
Corbett TH, Valeriote FA, Demchik L, Lowichik N, Polin L, Panchapor C, Pugh S, White K, Kushner J, Rake J, Wentland M, Golakoti T, Hetzel C, Ogino J, Patterson G, Moore R (1997) Discovery of cryptophycin-1 and BCN-183577: examples of strategies and problems in the detection of antitumor activity in mice. Invest New Drugs 15:207–218
Corbett TH, Valeriote FA, Demchik L, Polin L, Panchapor C, Pugh S, White K, Knight J, Jones J, Jones L, LoRusso P, Foster B, Wiegand RA, Lisow L, Golakoti T, Heltzel CE, Ogino J, Patterson GM, Moore RE (1996) Preclinical anticancer activity of cryptophycin-8. J Exp Ther Oncol 1:95–108
Shih C, Teicher BA (2001) Cryptophycins: a novel class of potent antimitotic antitumor depsipeptides. Curr Pharm Des 7:1259–1276
Panda D, Himes RH, Moore RE, Wilson L, Jordan MA (1997) Mechanism of action of the unusually potent microtubule inhibitor cryptophycin 1. Biochemistry 36:12948–12953
Panda D, DeLuca K, Williams D, Jordan MA, Wilson L (1998) Antiproliferative mechanism of action of cryptophycin-52: kinetic stabilization of microtubule dynamics by high-affinity binding to microtubule ends. Proc Natl Acad Sci U S A 95:9313–9318
Panda D, Ananthnarayan V, Larson G, Shih C, Jordan MA, Wilson L (2000) Interaction of the antitumor compound cryptophycin-52 with tubulin. Biochemistry 39:14121–14127
Polin L, Valeriote F, White K, Panchapor C, Pugh S, Knight J, LoRusso P, Hussain M, Liversidge E, Peltier N, Golakoti T, Patterson G, Moore R, Corbett TH (1997) Treatment of human prostate tumors PC-3 and TSU-PR1 with standard and investigational agents in SCID mice. Invest New Drugs 15:99–108
Smith AB 3rd, Cho YS, Zawacki LE, Hirschmann R, Pettit GR (2001) First generation design, synthesis, and evaluation of azepine-based cryptophycin analogues. Org Lett 3:4063–4066
Shih C, Gossett LS, Gruber JM, Grossman CS, Andis SL, Schultz RM, Worzalla JF, Corbett TH, Metz JT (1999) Synthesis and biological evaluation of novel cryptophycin analogs with modification in the beta-alanine region. Bioorg Med Chem Lett 9:69–74
Varie DL, Shih C, Hay DA, Andis SL, Corbett TH, Gossett LS, Janisse SK, Martinelli MJ, Moher ED, Schultz RM, Toth JE (1999) Synthesis and biological evaluation of cryptophycin analogs with substitution at C-6 (fragment C region). Bioorg Med Chem Lett 9:369–374
Subbaraju GV, Golakoti T, Patterson GM, Moore RE (1997) Three new cryptophycins from Nostoc sp. GSV 224. J Nat Prod 60:302–305
Corbett T, Valeriote F, Moore R, Tius M, Barrow R, Hemscheidt T, Liang J, Paik S, Polin L, Pugh S, Kushner J, Harrison S, Shih J, Martinelli M (1997) Preclinical antitumor activity of cryptophycin-52/55 (C-52, C-55) against mouse tumors. Proc Am Assoc Cancer Res 38:225
Polin L, Valeriote F, Moore R, Tius M, Barrow R, Hemscheidt T, Liang J, Paik S, White K, Harrison S, Shih J, Martinelli M, Corbett T (1997) Preclinical antitumor activity of cryptophycin-52/55 (C-52, C-55) against human tumors in SCID mice. Proc Am Assoc Cancer Res 38:225
Corbett TH, Polin L, Roberts BJ, Lawson AJ, Leopold WR III, White K, Kushner J, Paluch J, Hazeldine S, Moore R, Rake J, Horwitz JP (2002) Transplantable syngeneic rodent tumors: solid tumors of mice. In: Teicher B (ed) Tumor models in cancer research. Humana Press, Totowa, pp 41–71
Corbett T, Valeriote F, LoRusso P, Polin L, Panchapor C, Pugh S, White K, Knight J, Demchik L, Jones J, Jones L, Lowichik N, Biernat L, Foster B, Wozniak A, Lisow L, Valdivieso M, Baker L, Leopold W, Sebolt J, Bissery M-C, Mattes K, Dzubow J, Rake J, Perni R, Wentland M, Coughlin S, Shaw JM, Liverside G, Liversidge E, Bruno J, Sarpotdar P, Moore R, Patterson G (1995) Tumor models and the discovery and secondary evaluation of solid tumor active agents. Int J Pharmacognosy [Suppl] 33:102–122
Foster BJ, Fortuna M, Media J, Wiegand RA, Valeriote FA (1998) Cryptophycin 1 cellular levels and effects in vitro using L1210 cells. Invest New Drugs 16:199–204
Noe DA (1998) Noncompartmental pharmacokinetic analysis. In: Grochow LB, Ames MM (eds) A clinician's guide to chemotherapy pharmacokinetics and pharmacodynamics. Lippincott, Williams & Wilkins, Baltimore, pp 515–530
Schultz RM, Shih C, Wood PG, Harrison SD, Ehlhardt WJ (1998) Binding of the epoxide cryptophycin analog, LY355703 to albumin and its effect on in vitro antiproliferative activity. Oncol Rep 5:1089–1094
Kessel D (1999) Protein-binding patterns of the antitumor antibiotic cryptophycin 52 as measured with a two-phase partitioning system. J Chromatogr B Biomed Sci Appl 735:121–126
Gerweck LE (2000) The pH difference between tumor and normal tissue offers a tumor specific target for the treatment of cancer. Drug Resist Updat 3:49–50
Acknowledgements
We acknowledge the expert technical support by Joseph Kassab and Andrew Erickson, and assistance by Daniel Black. Supported by NIH grants U19-CA53001 and U01-CA62487.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boinpally, R.R., Polin, L., Zhou, SL. et al. Pharmacokinetics and tissue distribution of cryptophycin 52 (C-52) epoxide and cryptophycin 55 (C-55) chlorohydrin in mice with subcutaneous tumors. Cancer Chemother Pharmacol 52, 25–33 (2003). https://doi.org/10.1007/s00280-003-0621-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0621-0