Abstract
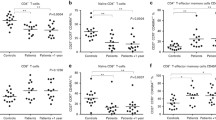
Multiple myeloma (MM) is a disease which remains incurable. One of the main reasons is a weakened immune system that allows MM cells to survive. Therefore, the current research is focused on the study of immune system imbalance in MM to find the most effective immunotherapy strategies. Aiming to identify the key points of immune failure in MM patients, we analysed peripheral lymphocytes subsets from MM patients (n = 57) at various stages of the disease course and healthy individuals (HI, n = 15) focusing on T, NK, iNKT, B cells and NK-cell cytokines. Our analysis revealed that MM patients exhibited immune alterations in all studied immune subsets. Compared to HI, MM patients had a significantly lower proportion of CD4 + T cells (19.55% vs. 40.85%; p < 0.001) and CD4 + iNKT cells (18.8% vs. 40%; p < 0.001), within B cells an increased proportion of CD21LCD38L subset (4.5% vs. 0.4%; p < 0.01) and decreased level of memory cells (unswitched 6.1% vs. 14.7%; p < 0.001 and switched 7.8% vs. 11.2%; NS), NK cells displaying signs of activation and exhaustion characterised by a more than 2-fold increase in SLAMF7 MFI (p < 0.001), decreased expression of NKG2D (MFI) and NKp46 (%) on CD16 + 56 + and CD16 + 56- subset respectively (p < 0.05), Effective immunotherapy needs to consider these immune defects and monitoring of the immune status of MM patients is essential to define better interventions in the future.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
No datasets were generated or analysed during the current study.
References
Kyle R, Rajkumar SV (2014) Erratum: Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 28(4):980. https://doi.org/10.1038/leu.2014.11
Pardoll DM (2015) Cancer and the immune system: Basic concepts and targets for intervention. Semin Oncol 42(4):523–538. https://doi.org/10.1053/j.seminoncol.2015.05.003
Altman J, Benavides AD, Das R, Bassiri H (2015) Antitumor responses of invariant natural killer T cells. J Immunol Res 2015:1–10. https://doi.org/10.1155/2015/652875
Smyth MJ, Hayakawa Y, Takeda K, Yagita H (2002) New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2(11):850–861. https://doi.org/10.1038/nrc928
Hiam-Galvez KJ, Allen BM, Spitzer MH (2021) Systemic immunity in cancer. Nat Rev Cancer 21(6):345–359. https://doi.org/10.1038/s41568-021-00347-z
Bos R, Sherman LA (2010) CD4 + T-Cell help in the tumor milieu is required for recruitment and cytolytic function of CD8 + T lymphocytes. Cancer Res 70(21):8368–8377. https://doi.org/10.1158/0008-5472.can-10-1322
Raskov H, Orhan A, Christensen JP, Gögenür I (2020) Cytotoxic CD8 + T cells in cancer and cancer immunotherapy. Br J Cancer 124(2):359–367. https://doi.org/10.1038/s41416-020-01048-4
Cooper MA, Colonna M, Yokoyama WM (2009) Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep 10(10):1103–1110. https://doi.org/10.1038/embor.2009.203
Groth A, Klöß S, Von Strandmann EP, Koehl U, Koch J (2011) Mechanisms of tumor and viral immune escape from natural killer cell-mediated surveillance. J Innate Immun 3(4):344–354. https://doi.org/10.1159/000327014
Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM (2015) NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol 6. https://doi.org/10.3389/fimmu.2015.00368
Díaz-Basabe A, Strati F, Facciotti F (2020) License to kill: when INKT cells are granted the use of lethal cytotoxicity. Int J Mol Sci 21(11):3909. https://doi.org/10.3390/ijms21113909
Kuylenstierna C, Björkström NK, Andersson S, Sahlström P, Bošnjak L, Paquin-Proulx D, Malmberg K, Ljunggren H, Moll M, Sandberg JK (2011) NKG2D performs two functions in invariant NKT cells: direct TCR‐independent activation of NK‐like cytolysis and co‐stimulation of activation by CD1d. Eur J Immunol 41(7):1913–1923. https://doi.org/10.1002/eji.200940278
Dosani T, Carlsten M, Marić I, Landgren O (2015) The cellular immune system in myelomagenesis: NK cells and T cells in the development of MM and their uses in immunotherapies. Blood Cancer J 5(4):e306. https://doi.org/10.1038/bcj.2015.32
Kay NE, Leong TL, Bone ND, Vesole DH, Greipp PR, Van Ness BG, Oken MM, Kyle RA (2001) Blood levels of immune cells predict survival in myeloma patients: results of an Eastern cooperative oncology group phase 3 trial for newly diagnosed multiple myeloma patients. Blood 98(1):23–28. https://doi.org/10.1182/blood.v98.1.23
Chan AC, Neeson PJ, Leeansyah E, Tainton KM, Quach H, Prince HM, Harrison SJ, Godfrey DI, Ritchie D, Berzins SP (2013) Natural killer T cell defects in multiple myeloma and the impact of lenalidomide therapy. Clin Exp Immunol 175(1):49–58. https://doi.org/10.1111/cei.12196
Yundi G, Jin Y, Ding J, Wu Y, Shi Q, Qu X, Zhao S, Li J, Chen L (2020) Low absolute CD4 + T cell counts in peripheral blood predict poor prognosis in patients with newly diagnosed multiple myeloma. Leuk Lymphoma 61(8):1869–1876. https://doi.org/10.1080/10428194.2020.1751840
Rubio MT, Dhuyser A, Nguyen S (2021) Role and modulation of NK cells in multiple myeloma. Hemato 2(2):167–181. https://doi.org/10.3390/hemato2020010
Wu S, Van Der Vliet HJ, Tai Y-T, Prabhala R, Wang R, Podar K, Catley L, Shammas MA, Anderson KC, Balk SP, Exley MA, Munshi NC (2008) Generation of antitumor invariant natural killer T cell lines in multiple myeloma and promotion of their functions via lenalidomide: a strategy for immunotherapy. Clin Cancer Res 14(21):6955–6962. https://doi.org/10.1158/1078-0432.ccr-07-5290
Spanoudakis E, Hu M, Naresh KN, Terpos E, Melo V, Reid A, Kotsianidis Ι, Abdalla S, Rahemtulla A, Karadimitris A (2009) Regulation of multiple myeloma survival and progression by CD1d. Blood 113(11):2498–2507. https://doi.org/10.1182/blood-2008-06-161281
Niu C, Jin H, Li M, Zhu S, Zhou L, Feng J, Zhou Y, Xu D, Xu J, Zhao L, Hao S, Li W, Cui J (2016) Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and γδ T cell-mediated lysis in multiple myeloma. Oncotarget 8(4):5954–5964. https://doi.org/10.18632/oncotarget.13979
Hosoya H, Sidana S (2021) Antibody-based treatment approaches in multiple myeloma. Curr Hematol Malig Rep 16(2):183–191. https://doi.org/10.1007/s11899-021-00624-6
Morandi F, Horenstein AL, Costa F, Giuliani N, Pistoia V, Malavasi F (2018) CD38: a target for immunotherapeutic approaches in multiple myeloma. Front Immunol 9. https://doi.org/10.3389/fimmu.2018.02722
Van De Donk NWCJ, Usmani SZ (2018) CD38 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front Immunol 9. https://doi.org/10.3389/fimmu.2018.02134
Romano A, Storti P, Marchica V, Scandura G, Notarfranchi L, Craviotto L, Di Raimondo F, Giuliani N (2021) Mechanisms of action of the new antibodies in use in multiple myeloma. Front Oncol 11. https://doi.org/10.3389/fonc.2021.684561
Pazina T, Macfarlane AW, Bernabei L, Dulaimi E, Kotcher RE, Yam C, Bezman N, Robbins M, Ross EA, Campbell KS, Cohen AD (2021) Alterations of NK cell phenotype in the disease course of multiple myeloma. Cancers 13(2):226. https://doi.org/10.3390/cancers13020226
Li X, Garg TK, Johnson S, Szmania S, Stivers J, Wen L, Khan S, Malaviarachchi PA, Barlogie B, Van Rhee F, Yaccoby S (2014) ATRA upregulates cell surface CD1D on myeloma cells and sensitizes them to INKT cell-mediated lysis. Blood 124(21):2102. https://doi.org/10.1182/blood.v124.21.2102.2102
Sullivan BA (2005) Activation or anergy: NKT cells are stunned by -galactosylceramide. J Clin Invest 115(9):2328–2329. https://doi.org/10.1172/jci26297
Miguel JFS, González M, Gascón A, Moro MJ, Jm H, Ortega F, Jiménez R, Guerras L, Romero MR, Casanova F (1992) Lymphoid subsets and prognostic factors in multiple myeloma. Br J Haematol 80(3):305–309. https://doi.org/10.1111/j.1365-2141.1992.tb08137.x
Klarquist J, Cross EW, Thompson SB, Willett B, Aldridge D, Caffrey-Carr AK, Xu Z, Hunter CA, Getahun A, Kedl RM (2021) B cells promote CD8 T cell primary and memory responses to subunit vaccines. Cell Rep 36(8):109591. https://doi.org/10.1016/j.celrep.2021.109591
Lavielle M, Mulleman D, Goupille P et al (2016) Repeated decrease of CD4 + T-cell counts in patients with rheumatoid arthritis over multiple cycles of rituximab treatment. Arthritis Res Ther 18:253. https://doi.org/10.1186/s13075-016-1152-5
Chawla S, Jindal AK, Arora K, Tyagi R, Dhaliwal M, Rawat A (2023) T cell abnormalities in X-Linked agammaglobulinaemia: an updated review. Clin Rev Allergy Immunol 65(1):31–42. https://doi.org/10.1007/s12016-022-08949-7
Cooke RE, Quinn KM, Quach H, Harrison S, Prince HM, Koldej R, Ritchie D (2020) Conventional treatment for multiple myeloma drives premature aging phenotypes and metabolic dysfunction in T cells. Front Immunol 11. https://doi.org/10.3389/fimmu.2020.02153
Kay NE, Leong T, Kyle RA, Greipp PR, Billadeau DD, Van Ness B, Bone ND, Oken MM (1997) Circulating blood B cells in multiple myeloma: analysis and relationship to circulating clonal cells and clinical parameters in a cohort of patients entered on the Eastern Cooperative Oncology Group Phase III E9486 clinical trial. Blood 90(1):340–345. https://doi.org/10.1182/blood.v90.1.340
Tsujimoto T, Lisukov IA, Huang N, Mahmoud MS, Kawano M (1996) Plasma cells induce apoptosis of pre-B cells by interacting with bone marrow stromal cells. Blood 87(8):3375–3383. https://doi.org/10.1182/blood.v87.8.3375.bloodjournal8783375
Hansmann L, Blum LK, Ju C, Liedtke M, Robinson WH, Davis MM (2015) Mass cytometry analysis shows that a novel memory phenotype B cell is expanded in multiple myeloma. Cancer Immunol Res 3(6):650–660. https://doi.org/10.1158/2326-6066.cir-14-0236-t
Wilbrink R, Spoorenberg A, Arends S, Van Der Geest KSM, Brouwer E, Bootsma H, Kroese FGM, Verstappen GM (2021) CD27-CD38lowCD21low B-Cells are increased in axial spondyloarthritis. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.686273
Reincke M, Payne K, Harder I, Strohmeier V, Voll R, Warnatz K, Keller B (2020) The antigen presenting potential of CD21low B cells. Front Immunol 11. https://doi.org/10.3389/fimmu.2020.535784
Rijal S, Kok J, Coombes C, Smyth L, Hourigan J, Jain S, Talaulikar D (2020) High proportion of anergic B cells in the bone marrow defined phenotypically by CD21(–/low)/CD38- expression predicts poor survival in diffuse large B cell lymphoma. BMC Cancer 20(1). https://doi.org/10.1186/s12885-020-07525-6
Zabel F, Fettelschoss A, Vogel M, Johansen P, Kündig TM, Bachmann MF (2017) Distinct T helper cell dependence of memory B-cell proliferation versus plasma cell differentiation. Immunology 150(3):329–342. https://doi.org/10.1111/imm.12688
Chahin M, Branham Z, Fox AD, Leurinda C, Keruakous AR (2022) Clinical considerations for immunoparesis in multiple myeloma. Cancers 14(9):2278. https://doi.org/10.3390/cancers14092278
Sanchez E, Gillespie A, Tang G, Ferros M, Harutyunyan NM, Vardanyan S, Gottlieb J, Li M, Wang CS, Chen H, Berenson JR (2016) Soluble B-Cell maturation antigen mediates Tumor-Induced immune deficiency in multiple myeloma. Clin Cancer Res 22(13):3383–3397. https://doi.org/10.1158/1078-0432.ccr-15-2224
Rahman ZSM, Manser T (2004) B cells expressing BCL-2 and a signaling-impaired BAFF-Specific receptor fail to mature and are deficient in the formation of lymphoid follicles and germinal centers. J Immunol 173(10):6179–6188. https://doi.org/10.4049/jimmunol.173.10.6179
De Jonge K, Tillé L, Lourenço J, Hajjami HM, Nassiri S, Racle J, Gfeller D, Delorenzi M, Verdeil G, Baumgaertner P, Speiser DE (2021) Inflammatory B cells correlate with failure to checkpoint blockade in melanoma patients. OncoImmunology 10(1). https://doi.org/10.1080/2162402x.2021.1873585
Carpenter EL, Mick R, Rech AJ, Beatty GL, Colligon TA, Rosenfeld MR, Kaplan DE, Chang K, Domchek SM, Kanetsky PA, Fecher LA, Flaherty KT, Schuchter LM, Vonderheide RH (2009) Collapse of the CD27 + B-Cell compartment associated with systemic plasmacytosis in patients with advanced melanoma and other cancers. Clin Cancer Res 15(13):4277–4287. https://doi.org/10.1158/1078-0432.ccr-09-0537
Pessoa de Magalhaes RJ, Vidriales M, Paiva B, Fernández-Giménez C, García-Sanz R, Mateos M, Gutiérrez NC, Lécrevisse Q, Blanco JF, Hernández J, De Las Heras N, Martínez‐López J, Roig MG, Da Costa ES, Ocio EM, Pérez‐Andrés M, Maiolino Â, Nucci M, De La Rubia J, Lahuerta J-J, Miguel JFS, Órfão A (2012) Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica 98(1):79–86. https://doi.org/10.3324/haematol.2012.067272
Orrantia A, Terrén Í, Astarloa-Pando G, González C, Uranga A, Mateos-Mazón JJ, García-Ruiz JC, Riñón M, Rey M, Pérez-Fernández S, Zenarruzabeitia O, Borrego F (2021) NK cell reconstitution after autologous hematopoietic stem cell transplantation: Association between NK cell maturation stage and outcome in multiple myeloma. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.748207
Dulphy N, Haas P, Busson M, Belhadj S, De Latour RP, Robin M, Carmagnat M, Loiseau P, Tamouza R, Scieux C, Rabian C, Di Santo JP, Charron D, Janin A, Socié G, Toubert A (2008) An unusual CD56brightCD16low NK Cell Subset dominates the early posttransplant period following HLA-Matched hematopoietic stem cell transplantation. J Immunol 181(3):2227–2237. https://doi.org/10.4049/jimmunol.181.3.2227
Besson L, Charrier E, Karlin L, Allatif O, Marçais A, Rouzaire P, Belmont L, Attal M, Lombard C, Salles G, Walzer T, Viel S (2018) One-year Follow-Up of natural killer cell activity in multiple myeloma patients treated with adjuvant lenalidomide therapy. Front Immunol 9. https://doi.org/10.3389/fimmu.2018.00704
Zambello R, Barilà G, Manni S, Piazza F, Semenzato G (2020) NK cells and CD38: implication for (Immuno)Therapy in plasma cell dyscrasias. Cells 9(3):768. https://doi.org/10.3390/cells9030768
Gars ML, Seiler C, Kay A, Bayless N, Solà E, Starosvetsky E, Moore L, Shen-Orr SS, Aziz N, Dekker CL, Khatri P, Swan GE, Davis MM, Holmes S, Blish CA (2018) CD38 contributes to human natural killer cell responses through a role in immune synapse formation. https://doi.org/10.1101/349084. bioRxiv (Cold Spring Harbor Laboratory)
Qian S, Xiong C, Wang M, Zhang Z, Fu Y, Hu Q, Ding H, Han X, Shang H, Jiang Y (2022) CD38 + CD39 + NK cells associate with HIV disease progression and negatively regulate T cell proliferation. Front Immunol 13. https://doi.org/10.3389/fimmu.2022.946871
Molfetta R, Quatrini L, Santoni A, Paolini R (2017) Regulation of NKG2D-Dependent NK cell functions: the Yin and the Yang of receptor endocytosis. Int J Mol Sci 18(8):1677. https://doi.org/10.3390/ijms18081677
Gutierrez-Guerrero A, Mancilla‐Herrera I, Maravillas-Montero JL, Martínez-Duncker I, Veillette A, Cruz-Muñoz ME (2021) SLAMF7 selectively favors degranulation to promote cytotoxicity in human NK cells. Eur J Immunol 52(1):62–74. https://doi.org/10.1002/eji.202149406
Wang R, Jaw J, Stutzman N, Zou Z, Sun PD (2011) Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol 91(2):299–309. https://doi.org/10.1189/jlb.0611308
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby RJ, Jonsson CB, Kanneganti T (2020) Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. bioRxiv. https://doi.org/10.1101/2020.10.29.361048. Cold Spring Harbor Laboratory)
Jourdan M, Tarte K, Legouffe E, Brochier J, Rossi JF, Klein B (1999) Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur Cytokine Netw 10(1):65–7055
Bradley JR (2007) TNF-mediated inflammatory disease. J Pathol 214(2):149–160. https://doi.org/10.1002/path.2287
Handa H, Murakami Y, Ishihara R, Kimura-Masuda K, Masuda Y (2019) The role and function of microRNA in the pathogenesis of multiple myeloma. Cancers 11(11):1738. https://doi.org/10.3390/cancers11111738
Sun Z, Shi K, Yang S, Wang Z, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W (2018) Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer 17(1). https://doi.org/10.1186/s12943-018-0897-7
Zhu YX, Kortuem KM, Stewart AK (2012) Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma 54(4):683–687. https://doi.org/10.3109/10428194.2012.728597
Mah AY, Cooper MA (2016) Metabolic regulation of natural killer cell IFN-Γ production. Crit Rev Immunol 36(2):131–147. https://doi.org/10.1615/critrevimmunol.2016017387
Nanbakhsh A, Malarkannan S (2021) The role of microRNAs in NK cell development and function. Cells 10(8):2020. https://doi.org/10.3390/cells10082020
Leong J, Wagner JA, Ireland AR, Fehniger TA (2017) Transcriptional and post-transcriptional regulation of NK cell development and function. Clin Immunol 177:60–69. https://doi.org/10.1016/j.clim.2016.03.003
Jin F, Du Z, Tang Y, Wang L, Yang Y (2019) Impact of microRNA29b on natural killer cells in Tcell acute lymphoblastic leukemia. Oncol Lett. https://doi.org/10.3892/ol.2019.10559
Mohsin A, Hussein T, Mashkoor KT (2021) The role of IL-2 in the pathogenesis of multiple myeloma a study in Iraqi patients. Kufa Med J 16(2):30–37. https://doi.org/10.36330/kmj.v16i2.1958
Böttcher J, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Sousa CRE (2018) NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment promoting Cancer Immune Control. Cell 172(5):1022–1037e14. https://doi.org/10.1016/j.cell.2018.01.004
Valiathan R, Ashman M, Asthana D (2016) Effects of Ageing on the Immune System: infants to Elderly. Scand J Immunol 83(4):255–266. https://doi.org/10.1111/sji.12413
Yan J, Greer JM, Hull R, O’Sullivan JD, Henderson RD, Read SJ, McCombe PA (2010) The effect of ageing on human lymphocyte subsets: comparison of males and females. Immunity & ageing: I & A, 7, 4. https://doi.org/10.1186/1742-4933-7-4
Almeida-Oliveira A, Smith-Carvalho M, Porto LC, Cardoso-Oliveira J, Ribeiro AS, Falcão RR, Abdelhay E, Bouzas LF, Thuler LC, Ornellas MH, Diamond HR (2011) Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol 72(4):319–329. https://doi.org/10.1016/j.humimm.2011.01.009
Funding
The study was supported by the grant of the Ministry of Health of the Czech Republic - Conceptual Development of Research Organization (Faculty Hospital in Pilsen—FNPl, 00669806), by Charles University Research Project No SVV 260 651,by the programme Cooperatio, research area ONCO and student’s project GAUK No 218522.
Author information
Authors and Affiliations
Contributions
Project administration: T.D, M.H.,; methodology: H.G., T.V, D.M, R.K., and A.J.; resources: D.L., M.H.; investigation: T.D., M.H.; data analysis: M.L., P.O., supervision: M.H., V.C., P.J.; figures preparation: M.C., M.L., T.V; writing: T.D. and M.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and under the World Marrow Donor Association standards. The ethics committee approved the study (joint committee of the Faculty of Medicine in Pilsen and Faculty Hospital Pilsen) on the 17th of June 2020 (reference number 295/2020). Informed consent for the study inclusion and results publication was obtained from all subjects involved in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dekojová, T., Gmucová, H., Macečková, D. et al. Lymphocyte profile in peripheral blood of patients with multiple myeloma. Ann Hematol 103, 5615–5625 (2024). https://doi.org/10.1007/s00277-024-05820-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05820-x