Abstract
Marginal zone lymphoma (MZL) is an uncommon subtype of non-Hodgkin lymphoma (NHL). Combination of rituximab and cladribine (R-2CdA) is a potential option for indolent NHL (iNHL) and mantle cell lymphoma (MCL) patients. The goal of this multicenter retrospective study was to assess the efficacy and safety of R-2CdA in MZL to support consensus-reaching in first-line therapy in advanced-stage patients. We searched electronic medical records databases of eight centers in China. Between November 2014 and December 2019, 183 symptomatic advanced MZL patients (42 treated with R-2CdA and 141 with rituximab plus cyclophosphamide, adriamycin, vincristine, and prednisone [R-CHOP]) were identified. After propensity score matching (PSM) (1:1) to adjust for clinical characteristics, 39 patients from each treatment arm were selected. The overall response rate (ORR) (84.6% vs. 94.9%, P = 0.263) and complete response rate (59.0% vs. 66.7%, P = 0.487) were comparable between two protocols. Neither progression-free survival (PFS), including the 5-year PFS (67.7% vs. 56.1%, P = 0.352), nor overall survival was improved by R-2CdA versus R-CHOP. However, R-2CdA was more tolerable than R-CHOP in MZL patients regarding grade 3/4 hematological adverse events (odds ratio [OR] 0.565, 95% confidence interval [CI] neutropenic fever (OR 0.795, 95% CI 0.678–0.932), and infections (OR 0.800, 95% CI 0.640–1.000). Overall, our study demonstrated that R-2CdA is potentially as effective as but safer than R-CHOP in advanced MZL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marginal zone lymphoma (MZL) is a relatively rare type among non-Hodgkin lymphomas (NHL), accounting for 5–8% of B-cell lymphomas in western countries [1, 2] and about 12.5% among Chinese counterparts [3]. MZL can be subclassified into extranodal MZL of mucosa-associated lymphoid tissue (MALT lymphoma), splenic and nodal MZLs (SMZL, NMZL), comprising 50–70%, 20%, and 10% of MZLs, respectively [4]. MALT lymphoma is more prevalent in females and has various clinical manifestations depending on the localization of tumor lesions. Among others, gastric MALT lymphoma is a more common subtype accounting for about one-third of MZLs than those in ocular adnexa, salivary glands, lungs, thyroid, and intestines containing abundant lymphoid tissue. MALT lymphoma can be triggered by infectious pathogens and autoimmune disorders [4,5,6]. Most MALT lymphomas are localized and about 20% are disseminated. Bone marrow involvement is rare in this patient group but is common in NMZL patients with peripheral lymphadenopathy. Peripheral cytopenia and splenomegaly are indicators of SMZL.
Estimates based on the Surveillance, Epidemiology, and End Results (SEER) database analysis reveal high 5-year survival rates of MALT lymphoma, SMZL, and NMZL, of 88.7%, 79.7%, and 76.5%, respectively[1]. However, whether Chinese MZL patients enjoy a long-term prognosis remains unknown. Most early-stage (Ann Arbor stage I/II) patients can be cured by local treatment with antibiotics (for gastric MALT lymphoma with Helicobacter pylori (HP) infection or SMZL with hepatitis C virus infection), radiotherapy (for localized MALT lymphomas), or surgical resection (for breast or thyroid MALT lymphomas and SMZL) [7,8,9]. But symptomatic advanced MALT lymphomas are currently considered incurable and often require systemic immunochemotherapy. However, a consensus on a first-line strategy for advanced patients has not yet been confirmed. A combination of rituximab and other chemotherapy agents such as chlorambucil, bendamustine, or anthracyclines has been ascertained to be effective and tolerable in patients with MZL [10,11,12]. But random control trials for stronger evidence for advanced MZL are urgently required and should be conducted more efficiently given the limited number of MZL cases.
Cladribine (2CdA) is a purine nucleoside analogue that has been used for hairy cell leukemia (HCL) and shows high efficacy and favorable acute and long-term toxicity [13]. Its resistance to cellular catabolism makes it equally toxic to dividing and non-dividing cells via diverse mechanisms, thus highly active in indolent lymphoproliferative diseases [14]. Cladribine has been shown to reverse immunosuppression and reduce the number of infective episodes in low-grade lymphomas [15, 16]. Jager et al. conducted a long-term follow-up of a prospective phase II trial of 2CdA for gastric MALT lymphoma (n = 26) and found that it offered excellent tumor control with long-lasting remission of the disease [17]. Recently, a single-center retrospective study (n = 49) with a median follow-up of 61 months confirmed the potential of 2CdA to induce durable remissions of MALT lymphoma [18].
In this multicentral retrospective study, comparing rituximab plus 2CdA (R-2CdA) with rituximab plus cyclophosphamide, adriamycin, vincristine, and prednisone (R-CHOP) regimen, we assessed the efficacy and safety of rituximab plus 2CdA (R-2CdA) in Chinese patients with advanced MZL. Propensity score matching (PSM) was utilized to match subjects between the two regimen groups.
Materials and methods
Patients
We retrospectively researched electronic medical records databases of eight participating lymphoma centers (one from Qingdao, one from Haikou, and six centers from Shanghai) in China. Between November 2014 and December 2019, 333 symptomatic MZL patients who had received systemic chemotherapy according to GELF [19] principle as a first-line regimen at these centers were identified. Among them, 183 patients, comprising 42 treated with R-2CdA and 141 with R-CHOP, were included. All patients enrolled were pathologically diagnosed by board-certified hematological pathologists from Zhongshan Hospital of Fudan University and the Affiliated Hospital of Qingdao University based on the 2016 World Health Organization classification criteria for lymphoid neoplasms [20].
Clinicopathological data obtained from the eight databases were as follows: age at diagnosis, gender, B-symptoms, Eastern Cooperative Oncology Group performance status (ECOG PS) scores, Ann Arbor stage, the site of external, nodal, or bone marrow involvement, autoimmune conditions such as the presence of autoantibodies and a history of autoimmune disorders, and levels of lactate dehydrogenase (LDH), β2-microglobulin (β2MG), hemoglobin (Hb), platelet (PLT), D-dimer, CD5, and Kiel-67 antigen (Ki-67).
Treatment
All patients received a 28-day cycle R-2CdA regimen consisting of rituximab 375 mg/m2 IV day 1 and cladribine 0.1 mg/kg IV days 1–4 of each cycle. The R-CHOP regimen was administered every 3 weeks in a maximum of 8 courses, comprised of cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2 on day 1, and prednisone 100 mg/day for 5 days of each 21-day circle. Rituximab maintenance treatment was not given.
Efficacy and safety follow-up
Treatment response was assessed using the 2007 Revised Response Criteria for Malignant Lymphoma after 2–4 cycles of therapy and completion of scheduled therapy [21]. Treatment-related adverse events were counted, including peripheral cytopenia, neutropenic fever, infection, venous thromboembolism, organ damage, and so forth. Toxicity was graded according to the National Cancer Institute’s Common Toxicity Criteria for Adverse Events v5.0.
The study was conducted in accordance with the Declaration of Helsinki. Oral informed consent was obtained from all participants during outpatient or telephone interviews for the utility of the anonymized medical data for research. The study protocol and informed consent form were approved by the clinical ethics committee of Zhongshan Hospital of Fudan University, the lead site of this study.
Statistical analysis
Categorical variables related to clinical characteristics were reported in counts and percentages, and the continuous variables were expressed as median (range). The primary endpoint was the overall response rate (ORR) of patients treated with the two first-line immunochemotherapy regimens, with ORR being defined as the proportion of patients sent back complete response (CR) or partial response (PR). Secondary outcomes included progression-free survival (PFS), overall survival (OS), and complications related to therapy, with PFS being defined as the time from diagnosis to disease progression/relapse or death of any cause. Overall survival (OS) was calculated from date of diagnosis to date of death or last follow-up. The following variables were prognostic predictors for survival in MZL patients, including age, gender, ECOG PS score, B-symptoms, Ann Arbor stage, autoimmune disease (AID), anemia, thrombocytopenia, elevated LDH, elevated β2MG, abnormal D-dimer, bone marrow involvement, six or more areas of nodal involvement (LN6), and extranodal involvement in two or more organs (MMS2).
To compare the efficiency and safety between R-2CdA and R-CHOP regimen with minimal effects of potential confounders and selection bias, we used 1:1 PSM to match the participants of the two regimen groups, which was performed using the nearest-neighbor method with a caliper size of 0.05 by logistic regression analysis. All statistical analyses were performed using SPSS version 23.0. Survival analysis was performed on PFS and OS using the Kaplan–Meier method with the log-rank test for comparisons, and results were expressed as rates by year and 95% CIs. Differences between groups were estimated with the χ2 test or Fisher’s exact test as appropriate. A p-value of < 0.05 indicated statistical significance. The Cox proportional hazards model with a backward stepwise variable selection was utilized for multivariate regression analysis.
Results
Clinical characteristics of MZL patients
The characteristics of 183 MZL patients treated with R-2CdA or R-CHOP as the first-line regimen are detailed in Table 1. The median age at diagnosis was 58 (range 28 to 80). A total of 130 (71.0%) patients were with advanced stage and 67 (36.6%) patients were with B symptoms. Bronchial-associated lymphoid tissue (BALT) lymphoma was predominant (34.6%) in all cases, followed by gastric MALT lymphoma (25.6%), NMZL (5.1%), and SMZL (2.6%).
Prognostic outcomes before PSM
The 183 patients had a median follow-up of 51.4 months and median PFS of 98.4 months (95% confidence interval [CI] 1061.7 to NA), and median OS was not reached (Fig. 1A and B). PFS was not significantly influenced by gender, age (> 70 years old), B-symptoms, presence of AID, thrombocytopenia (PLT < 100 × 109/L), or CD5 expression. However, ECOG PS score of ≥ 2 points, Ann Arbor stage III/IV, anemia (Hb < 120 g/mL), serum LDH of > 245 mg/mL, serum β2-MG of > 2.8 mg/mL, D-dimer abnormality, bone marrow involvement, more than two sites of extranodal involvement (MMS2), more six areas of nodal involvements (LN6), and Ki67 of ≥ 20% were negatively associated with poor PFS. Multivariate Cox logistic regression analysis showed that ECOG performance, D-dimer, and LN6 were risk factors for relapse/progression and survival in MZL. Neither PFS nor OS was improved by chemotherapy regimen selection (Table 2 and Fig. 1C–H).
Kaplan–Meier analysis of progression-free survival and overall survival (panels A to B, n = 183) in patients before PSM, Kaplan–Meier analysis of progression-free survival and overall survival by ECOG PS ≥ 2 (panels C to D, n = 183) in patients before PSM, Kaplan–Meier analysis of progression-free survival and overall survival by D-dimer (panels E to F, n = 183) in patients before PSM, Kaplan–Meier analysis of progression-free survival and overall survival by LN ≥ 6 (panels G to H, n = 183) in patients before PSM, and Kaplan–Meier analysis of progression-free survival and overall survival by regimen (panels I to J, n = 183) in patients before PSM
Clinical characteristics after PSM
After propensity score matching (PSM) (1:1) to adjust for clinical characteristics, 39 patients from each treatment arm were selected. There were no differences in ECOG PS score, presentation of B symptoms, and elevated β2MG after PSM, which were significantly discrepant before PSM. Most patients (71.8% in the R-2CdA group and 69.2% in the R-CHOP group) were diagnosed with advanced MZL. 46.2% of the patients in 2 arms presented with systemic symptoms(P = 1.0) and 56.4% and 48.7% of patients had an ECOG PS score of ≥ 2 in R-2CdA and R-CHOP groups, respectively(P = 0.496). Elevated D-dimer was detectable in 25.6% versus 28.2% (P = 0.799) of samples from patients receiving R-2CdA and R-CHOP, respectively (Table 1).
Response and survival after PSM
The median number of immunochemotherapy cycles received was six (range 3–8). 92.3% of patients (84.7% in R-2CdA and 94.9% in R-CHOP, P = 0.675) were treated with the two regimens for four or more cycles, without significant difference between the two regimens. There were 6 gastric MALT patients in the RC group, among whom 4 patients were HP-negative at diagnosis, one patient was positive, and one patient’s HP status was unknown. The positive one received RC regimen and anti-HP treatment simultaneously. At the same time, 14 gastric MALT patients were in the R-CHOP group, where 7 patients failed to respond to anti-HP treatment and the others were unclear about their HP status.
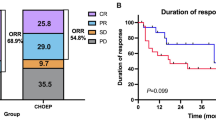
Among all patients, the ORR was 89.7% with 62.8% CR/CRu. There were no differences in ORR (84.6% vs. 94.9%, P = 0.263) and CR rate (59.0% vs. 66.7%, P = 0.482) between R-2CdA and R-CHOP, nor were there any differences (ORR 95.0% vs. 87.9%, P = 0.672; CR rate 70.0% vs. 60.3%, P = 0.441) observed between gastric and non-gastric MALT. The CR rates were comparable, of 61.7% and 64.5%, between gastric MALT versus BALT lymphoma (P = 0.81). However, it was higher for patients with stage I/II than that for advanced-stage individuals (78.3% vs. 54.5%, P = 0.05) (Table 3).
A median follow-up of 34.7 (range 5 to 108.7 months) versus 28.5 months (range 4.9 to 144.9 months) was found for the R-2CdA and R-CHOP cohorts. The mean duration from diagnosis to initiation of chemotherapy in 2 groups was comparable (32 vs 40 days, P = 0.235). Median OS and PFS were not reached for those receiving R-2CdA or R-CHOP (Fig. 2A to B). Five (12.8%) R-2CdA-treated patients experienced progression of disease within 24 months (POD24), compared to seven (17.9%) R-CHOP-treated ones (P = 0.53). PFS rates at 5 years were 67.7% ± 11.2% in R-2CdA and 56.1% ± 12.1% in R-CHOP (Table 3 and Fig. 2C). The OS rate of patients remained constant without difference between R-2CdA and R-CHOP, with 5-year OS rates of 93.1% ± 4.7% and 94.7% ± 3.7%, respectively (Table 4 and Fig. 2D).
While several rescue regimens were included (R-CHOP, rituximab plus gemcitabine and oxaliplatin [R-GemOx], ifosfamide, carboplatin and etoposide [22], BR, R2, ibrutinib plus rituximab, phosphoinositide 3-kinase inhibitor [PI3Ki] plus rituximab, and radiotherapy), patient response to them was poor. All the R-2CdA-treated patients (n = 8) responded to the second-line regimen, and only 4/10 R-CHOP-treated participants to rescue therapy (P = 0.013). There was no histologically transformed tumor after relapse/progression.
Safety
Among the 78 patients selected, 125 adverse events (AEs) were recorded in 51 patients (65.4%), including 47 grade 3/4 AEs and one serious AE (SAE). In the R-2CdA arm, grade 1/2 AEs were predominant (87.5%). The most frequent hematological AEs was neutropenia (n = 18, 62.1%). Non-hematological AEs comprised of pulmonary infection, rash, herpes zoster, enterobrosis, vein thrombosis, renal impairment, and impaired olfaction. Among patients treated with R-CHOP, grade 3/4 AEs accounted for 49.4%, of which neutropenia was the most frequent hematological AE and its associated AEs such as infectious fever (n = 6), pulmonary infection (n = 2), and cytomegalovirus (CMV) infection were frequently reported, which required in-patient hospitalization (Table 5). R-2CdA-treated patients suffered less toxicity than R-CHOP-treated controls in terms of grade 3/4 hematological AEs (odds ratio [OR] 0.565, 95% CI 0.411 to 0.777), neutropenic fever (OR 0.795, 95% CI 0.678 to 0.932), and infections (OR 0.8, 95% CI 0.64 to 1.0) (Table 5). The only SAE was grade 3 enterobrosis, reported in a 59-year old female patient with the primary lesion located in lung. After the first cycle of R-2CdA, she presented with acute abdominal pain caused by jejunal perforation associated with MALT lymphoma involvement, which was confirmed by excisional biopsy. Nevertheless, this participant achieved complete remission after four cycles of R-2CdA regimen and bowel resection procedure, though delayed due to the SAE. No treatment-related death was observed in both arms.
Discussion
MZL is a common type of indolent NHL. Given its heterogeneity, it is challenging to define a widely accepted treatment principle for these patients. For localized involvement, appropriate local therapy comprises antibiotics against H. pylori for gastric MALT lymphoma, splenectomy for SMZL, and radiotherapy for NMZL. But appropriate regimens for advanced-stage patients with low tumor burden or asymptomatic MZL must still be determined. Moreover, the debate over first-line chemotherapy regimens in the treatment of advanced and symptomatic MZL remains unsolved today.
The IELSG-19 study [10] is the largest phase III randomized trial of frontline MALT lymphoma, in which patients were randomized to chlorambucil monotherapy, rituximab monotherapy, or the combination of rituximab and chlorambucil. The ORR was 94.7% (78.8% CR) in patients receiving the combined therapy, significantly higher than the percentage in those receiving either monotherapy. The 5-year PFS rate was 72% (95% CI 63 to 79) in patients treated with combination therapy, superior to the other two groups. These rates are better than our results in the R-2CdA and R-CHOP groups. Furthermore, the 5-year OS in the IELSG-19 trial was similar in the three arms: 89% in chlorambucil, 92% in rituximab, and 90% in the combination arm, which is comparable to our results. Several reasons may explain the difference in outcomes between our results and those achieved from the IELSG-19 study. Participants in the IELSG-19 study had a less severe cancer stage at diagnosis: 43.6% of them at advanced stage, only 1.5% of patients with an ECOG PS score ≥ 2, and 42.6% with primary gastric MALT lymphoma. The International Prognostic Index (IPI) categorized 80.7% of patients as low or low-intermediate risk. Furthermore, the IELSG-19 study was a prospective trial and patients were managed more carefully and standardly. Moreover, our study and the IELSG-19 study showed no difference in OS, probably attributed to the indolent progression of MZL and rescue regimens that may overlap and intersect.
The BRIGHT study, a multicenter phase III randomized study, compared the efficacy of BR vs. R-CHOP/R-CVP in patients with untreated iNHL or mantle-cell lymphoma (MCL), and the sample size of MZLs was 46 [12]. The BRIGHT study showed the difference in the 5-year PFS rate between BR and R-CHOP regimens (65.5% vs. 55.8%), which was significant for all participants. However, the subgroup analysis of MZL failed to show a trend in favor of BR (P = 0.0582). The BRIGHT study reported similar 5-year PFS rates in the R-CHOP/R-CVP group to ours. Furthermore, both studies failed to show a significant difference in PFS rates between groups, probably due to the small sample size and retrospective nature.
Development of MALT lymphoma is strongly associated with chronic antigenic drives and autoimmune disorders, which makes it suitable for immunomodulatory treatment. Recently, a phase 2 investigator-initiated study enrolled 30 previously untreated III/IV stage MZL patients. They were treated with 6 or 12 cycles regimen combining lenalidomide with rituximab (R2) and achieved a robust efficacy with no unexpected toxicities. The ORR was 93% (CR/CRu 70%) and the median PFS was 59.8 months [23]. In addition, the Bruton’s kinase inhibitor ibrutinib and the proteasome inhibitor bortezomib also showed their efficacy among R/R MZL patients [24, 25].
Histological transformation of MZL was undetected in patients who progressed during the study period. However, previous studies [11, 12] reported an incidence of such transformation of 2 to 3%. This may be due to the small sample size and short-term follow-up. Besides, these studies included patients with follicular lymphoma (FL), an iNHL subtype more likely to transform to an aggressive tumor than MZL.
MZL is considered incurable and tends to occur in elderly individuals. AEs or complications related to treatment are critical for decision-making in choosing a chemotherapy regimen as elderly patients are unable to tolerate cytotoxic chemotherapy due to decreased organ function and comorbidities. Consistent with the results of other publications [16,17,18] that R-2CdA was associated with fewer short-term AEs, in our analysis, there were lower risks of grade 3/4 hematological AEs, neutropenic fever, and infectious complications that required patient hospitalization in R-2CdA versus R-CHOP. Previous studies have demonstrated that cladribine may be associated with an increased risk of myelodysplastic syndrome (MDS) secondary to indolent B cell lymphoma [26, 27]. In the present work, though no secondary tumors were detected in both groups, any questionable manifestations require longer surveillance and re-biopsy confirmation. However, the BRIGHT study [12] reported more new cancer diagnoses in patients treated with BR than those with R-CHOP/R-CVP, which cannot be interpreted correctly.
To our knowledge, this is the first study to evaluate the efficacy and safety of R-2CdA versus R-CHOP in the real-world setting. We utilized a propensity score matching method to control for potential confounders or predictors. This retrospective multicenter study showed non-inferiority of R-2CdA versus R-CHOP as first-line treatment for MZL patients. R-2CdA also showed less toxicity than R-CHOP, evidenced by low frequencies of grade 3/4 hematological AEs, neutropenic fever, and infections. Recently, a study reported that subcutaneous cladribine shows excellent long-term survival in patients with hairy cell leukemia [28]. Subcutaneous cladribine combining with rituximab could be an efficient and feasible choice for patients with MZL in future.
The limitations are apparent including the retrospective design and the fact that multiple centers are involved. Institutional bias is present in selecting chemotherapy regimens. Secondly, the sample is not enough to assess the efficacy and safety of these two treatment strategies for each entity, especially for NMZL and SMZL. Moreover, the follow-up period of our study was relatively short for this indolent disease. Longer follow-up data and robust study protocols are needed to observe treatment-related complications like secondary malignancy.
Overall, this multicenter, large-sample, retrospective study indicates that excellent ORR (84.6% and 94.9%) and 5-year PFS (67.7% and 56.1%) can be achieved after either R-2CdA or R-CHOP in advanced-stage, treatment-naive patients with MZL. R-2CdA is more tolerable than R-CHOP in terms of grade 3/4 AEs. R-2CdA could be an initial treatment option regarding the efficacy and acceptable toxicity.
References
Olszewski AJ, Castillo JJ (2013) Survival of patients with marginal zone lymphoma: analysis of the Surveillance, Epidemiology, and End Results database. Cancer 119(3):629–638
(1997) A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 89(11):3909–3918
Xiao-qiu L, Gan-di L, Zi-fen G, Xiao-ge Z, Xiong-zeng Z (2012) Group tCLS: Distribution of lymphoma subtypes in China: analysis of 10002 multicentric cases in China. J Diagn Concepts Pract 11(2):5
Sindel A, Al-Juhaishi T, Yazbeck V (2019) Marginal zone lymphoma: state-of-the-art treatment. Curr Treat Options Oncol 20(12):90
Sriskandarajah P, Dearden CE (2017) Epidemiology and environmental aspects of marginal zone lymphomas. Best Pract Res Clin Haematol 30(1–2):84–91
Perrone S, D’Elia GM, Annechini G, Pulsoni A (2016) Infectious aetiology of marginal zone lymphoma and role of anti-infective therapy. Mediterr J Hematol Infect Dis 8(1):e2016006
Raderer M, Kiesewetter B, Ferreri AJ (2016) Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin 66(2):153–171
Iannitto E, Tripodo C (2011) How I diagnose and treat splenic lymphomas. Blood 117(9):2585–2595
Zucca E, Bertoni F (2016) The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood 127(17):2082–2092
Zucca E, Conconi A, Martinelli G, Bouabdallah R, Tucci A, Vitolo U, Martelli M, Pettengell R, Salles G, Sebban C et al (2017) Final results of the IELSG-19 randomized trial of mucosa-associated lymphoid tissue lymphoma: improved event-free and progression-free survival with rituximab plus chlorambucil versus either chlorambucil or rituximab monotherapy. J Clin Oncol 35(17):1905–1912
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C et al (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381(9873):1203–1210
Flinn IW, van der Jagt R, Kahl B, Wood P, Hawkins T, MacDonald D, Simpson D, Kolibaba K, Issa S, Chang J et al (2019) First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J Clin Oncol 37(12):984–991
Saven A, Burian C, Koziol JA, Piro LD (1998) Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood 92(6):1918–1926
Delannoy A (1996) 2-chloro-2′-deoxyadenosine: clinical applications in hematology. Blood Rev 10(3):148–166
Sigal DS, Saven A (2008) Cladribine in indolent non-Hodgkin’s lymphoma. Expert Rev Anticancer Ther 8(4):535–545
Wei Z, Li J, Cheng Z, Yuan L, Liu P (2017) A single center experience: rituximab plus cladribine is an effective and safe first-line therapy for unresectable bronchial-associated lymphoid tissue lymphoma. J Thorac Dis 9(4):1081–1092
Jäger G, Neumeister P, Quehenberger F, Wöhrer S, Linkesch W, Raderer M (2006) Prolonged clinical remission in patients with extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type treated with cladribine: 6 year follow-up of a phase II trial. Ann Oncol 17(11):1722–1723
Kiesewetter B, Dolak W, Simonitsch-Klupp I, Mayerhoefer ME, Raderer M (2017) Long-term safety and activity of cladribine in patients with extranodal B-cell marginal zone lymphoma of the mucosa-associated lymphoid tissue (MALT) lymphoma. Hematol Oncol 35(2):177–186
Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P et al (2011) Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 377(9759):42–51
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127(20):2375–2390
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Delfau-Larue MH, de Leval L, Joly B, Plonquet A, Challine D, Parrens M, Delmer A, Salles G, Morschhauser F, Delarue R et al (2012) Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica 97(10):1594–1602
Becnel MR, Nastoupil LJ, Samaniego F, Davis RE, You MJ, Green M, Hagemeister FB, Fanale MA, Fayad LE, Westin JR et al (2019) Lenalidomide plus rituximab (R(2) ) in previously untreated marginal zone lymphoma: subgroup analysis and long-term follow-up of an open-label phase 2 trial. Br J Haematol 185(5):874–882
Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, Collins GP, Ma S, Coleman M, Peles S et al (2017) Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 129(16):2224–2232
Conconi A, Martinelli G, Lopez-Guillermo A, Zinzani PL, Ferreri AJM, Rigacci L, Devizzi L, Vitolo U, Luminari S, Cavalli F et al (2011) Clinical activity of bortezomib in relapsed/refractory MALT lymphomas: results of a phase II study of the International Extranodal Lymphoma Study Group (IELSG). Ann Oncol 22(3):689–695
Jäger G, Höfler G, Linkesch W, Neumeister P (2004) Occurrence of a myelodysplastic syndrome (MDS) during first-line 2-chloro-deoxyadenosine (2-CDA) treatment of a low-grade gastrointestinal MALT lymphoma. Case report and review of the literature. Haematologica 89(4):Ecr01
Kroft SH, Tallman MS, Shaw JM, Thangavelu M, Peterson LC (1997) Myelodysplasia following treatment of chronic lymphocytic leukemia (CLL) with 2-chlorodeoxyadenosine (2-CdA). Leukemia 11(1):170
Benz R, Arn K, Andres M, Pabst T, Baumann M, Novak U, Hitz F, Hess U, Zenhaeusern R, Chalandon Y et al (2020) Prospective long-term follow-up after first-line subcutaneous cladribine in hairy cell leukemia: a SAKK trial. Blood Adv 4(15):3699–3707
Author information
Authors and Affiliations
Contributions
Conception and design: Peng Liu, Zheng Wei, Hongwei Xue. Management of patient and data collection: Yawen Wang, Jiadai Xu, Jing Li, Miaojie Shi, Rong Tao, Bobin Chen, Liuhua Sun, Yuyang Tian, Yunhua Hou, Qilin Zhan, Wenhao Zhang, Yan Ma. Pathological consultation: Jigang Wang. Data analysis and manuscript writing: Yawen Wang. Manuscript revising: Jiadai Xu, Peng Liu. Final approval of manuscript: Peng Liu, Hongwei Xue.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Xu, J., Li, J. et al. Rituximab plus cladribine versus R-CHOP in frontline management of marginal zone lymphoma in China: a propensity-score matched multicenter study. Ann Hematol 101, 2139–2148 (2022). https://doi.org/10.1007/s00277-022-04919-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04919-3