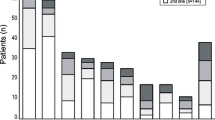
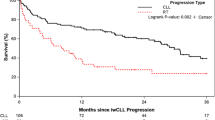
Abstract
Rituximab-containing chemotherapy remains a viable frontline treatment option for patients with chronic lymphocytic leukemia (CLL) in the era of novel agents. However, its effectiveness in the second-line setting—in relation to previous rituximab exposure in first-line—has hardly been evaluated in a population-based setting. Therefore, in this comprehensive, population-based study, we assessed the impact of first-line treatment with rituximab-containing chemotherapy on the effectiveness of second-line treatment with rituximab-containing chemotherapy. We selected all 1735 patients diagnosed with CLL between 2004 and 2010 from the Dutch Population-based HAematological Registry for Observational Studies (PHAROS). The primary endpoint was treatment-free survival (TFS). First- and second-line treatment was instituted in 663 (38%) and 284 (43%) patients, respectively. In first line, the median TFS was 19.7 and 67.1 months for chemotherapy without (n = 445; 67%) and with rituximab (n = 218; 33%), respectively (adjusted hazard ratio [HRadjusted], 0.83; P = 0.031). The median TFS among recipients of second-line chemotherapy without (n = 165; 57%) and with rituximab (n = 121; 42%) was 15.0 and 15.3 months, respectively (HRadjusted, 0.93; P = 0.614). Of the 121 patients who received rituximab-containing chemotherapy in second-line, 89 (74%) and 32 (26%) received first-line chemotherapy without and with rituximab, respectively. Median TFS in these two treatment groups was 18.3 and 12.1 months, respectively (HRadjusted, 1.71; P = 0.060). Collectively, in this population-based study, the effectiveness of first-line treatment with rituximab-containing chemotherapy was less pronounced in second-line treatment. The hampered effectiveness of rituximab-containing chemotherapy in second-line could not be explained by previous rituximab exposure.



Similar content being viewed by others
References
Van den Broek E, Kater A, van de Schans S, Karim-Kos H, Janssen-Heijnen M, Peters W et al (2012) Chronic lymphocytic leukaemia in the Netherlands: trends in incidence, treatment and survival, 1989–2008. Eur J Cancer 48(6):889–895
Kristinsson SY, Dickman PW, Wilson WH, Caporaso N, Björkholm M, Landgren O (2009) Improved survival in chronic lymphocytic leukemia in the past decade: a population-based study including 11,179 patients diagnosed between 1973–2003 in Sweden. haematologica. 94(9):1259–1265
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF et al (2016) Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374(4):311–322
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O'Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ, RESONATE-2 Investigators (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 373(25):2425–2437
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, Ghia P, Eradat H, Ervin T, Lamanna N, Coiffier B, Pettitt AR, Ma S, Stilgenbauer S, Cramer P, Aiello M, Johnson DM, Miller LL, Li D, Jahn TM, Dansey RD, Hallek M, O'Brien SM (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 370(11):997–1007
Hallek M (2017) Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol 92(9):946–965
Hallek M, Fischer K, Fingerle-Rowson G, Fink A, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grünhagen U, Bergmann M, Catalano J, Zinzani PL, Caligaris-Cappio F, Seymour JF, Berrebi A, Jäger U, Cazin B, Trneny M, Westermann A, Wendtner CM, Eichhorst BF, Staib P, Bühler A, Winkler D, Zenz T, Böttcher S, Ritgen M, Mendila M, Kneba M, Döhner H, Stilgenbauer S, International Group of Investigators, German Chronic Lymphocytic Leukaemia Study Group (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 376(9747):1164–1174
Delgado J, Baumann T, Ghita G, Montserrat E (2012) Chronic lymphocytic leukemia therapy: beyond chemoimmunotherapy. Curr Pharm Des 18(23):3356–3362
Shustik C, Bence-Bruckler I, Delage R, Owen CJ, Toze CL, Coutre S (2017) Advances in the treatment of relapsed/refractory chronic lymphocytic leukemia. Ann Hematol 96(7):1185–1196
Eichhorst B, Robak T, Montserrat E, Ghia P, Hillmen P, Hallek M et al (2015) Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(suppl_5):v78–v84
Smolewski P, Witkowska M, Korycka-Wołowiec A (2013) New insights into biology, prognostic factors, and current therapeutic strategies in chronic lymphocytic leukemia. ISRN Oncol 2013:7
group DBHCw (2016) Dutch guidelines for the diagnosis and treatment of chronic lymphocytic leukaemia. Neth J Med 74(2):68
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ, International Workshop on Chronic Lymphocytic Leukemia (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–working group 1996 guidelines. Blood. 111(12):5446–5456
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W et al (2018) Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 379(26):2517–2528
Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, Simkovic M, Samoilova O, Novak J, Ben-Yehuda D, Strugov V, Gill D, Gribben JG, Hsu E, Lih CJ, Zhou C, Clow F, James DF, Styles L, Flinn IW (2019) Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20(1):43–56
Fischer K, Al-Sawaf O, Bahlo J, Fink A-M, Tandon M, Dixon M et al (2019) Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 380(23):2225–2236
Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS et al (2017) Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol 18(2):230–240
Shanafelt TD, Borah BJ, Finnes HD, Chaffee KG, Ding W, Leis JF et al (2015) Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract 11(3):252–258
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, Opat S, Owen CJ, Samoylova O, Kreuzer KA, Stilgenbauer S, Döhner H, Langerak AW, Ritgen M, Kneba M, Asikanius E, Humphrey K, Wenger M, Hallek M (2014) Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370(12):1101–1110
Eichhorst B, Fink A-M, Bahlo J, Busch R, Kovacs G, Maurer C et al (2016) First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. The Lancet Oncology 17(7):928–942
Robak T, Dmoszynska A, Solal-Céligny P, Warzocha K, Loscertales J, Catalano J et al (2010) Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol 28(10):1756–1765
van der Straten L, Dinmohamed AG, Westerweel PE, Langerak AW, Riedl J, Doorduijn JK et al (2018) Rituximab addition to chemotherapy in real world patients with chronic lymphocytic leukemia: effective in first line but indication of lack of efficacy in subsequent lines of therapy. Leuk Lymphoma 59(11):2757–2761
Lee LJ, Toze CL, Huang SJ, Gillan TL, Connors JM, Sehn LH et al (2018) Improved survival outcomes with the addition of rituximab to initial therapy for chronic lymphocytic leukemia: a comparative effectiveness analysis in the province of British Columbia, Canada. Leuk Lymphoma 59(6):1356–1363
Takei K, Yamazaki T, Sawada U, Ishizuka H, Aizawa S (2006) Analysis of changes in CD20, CD55, and CD59 expression on established rituximab-resistant B-lymphoma cell lines. Leuk Res 30(5):625–631
Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri G-M, Bernasconi S, Tedesco F, Rambaldi A, Introna M (2000) Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 95(12):3900–3908
Marquez ME, Hernández-Uzcátegui O, Cornejo A, Vargas P, Da Costa O (2015) Bone marrow stromal mesenchymal cells induce down regulation of CD20 expression on B-CLL: implications for rituximab resistance in CLL. Br J Haematol 169(2):211–218
Schouten LJ, Höppener P, Van den Brandt PA, Knotternus AJ, Jager JJ (1993) Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol 22(3):369–376
Dinmohamed A, van Norden Y, Visser O, Posthuma E, Huijgens P, Sonneveld P, van de Loosdrecht A, Jongen-Lavrencic M (2015) Effectiveness of azacitidine for the treatment of higher-risk myelodysplastic syndromes in daily practice: results from the Dutch population-based PHAROS MDS registry. Leukemia. 29(12):2449–2451
Dinmohamed AG, van Norden Y, Visser O, Posthuma EFM, Huijgens PC, Sonneveld P, van de Loosdrecht A, Jongen-Lavrencic M (2015) The use of medical claims to assess incidence, diagnostic procedures and initial treatment of myelodysplastic syndromes and chronic myelomonocytic leukemia in the Netherlands. Leuk Res 39(2):177–182
Jaffe EHNSH, Vardinman JW (eds) (2001) World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Ann Oncol 13(3):490–491
Cramer P, Isfort S, Bahlo J, Stilgenbauer S, Döhner H, Bergmann M, Stauch M, Kneba M, Lange E, Langerbeins P, Pflug N, Kovacs G, Goede V, Fink AM, Elter T, Fischer K, Wendtner CM, Hallek M, Eichhorst B (2015) Outcome of advanced chronic lymphocytic leukemia following different first-line and relapse therapies: a meta-analysis of five prospective trials by the German CLL Study Group (GCLLSG). Haematologica. 100(11):1451–1459
Cheson B, Bennett J, Grever M, Kay N, Keating M, O'Brien S et al (1996) National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 87(12):4990–4997
Grambsch PM, Therneau TM (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 81(3):515–526
Sehn LH, Chua N, Mayer J, Dueck G, Trněný M, Bouabdallah K, Fowler N, Delwail V, Press O, Salles G, Gribben J, Lennard A, Lugtenburg PJ, Dimier N, Wassner-Fritsch E, Fingerle-Rowson G, Cheson BD (2016) Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol 17(8):1081–1093
Chaoui D, Choquet S, Sanhes L, Mahé B, Hacini M, Fitoussi O, Arkam Y, Orfeuvre H, Dilhuydy MS, Barry M, Jourdan E, Dreyfus B, Tempescul A, Leprêtre S, Bardet A, Leconte P, Maynadié M, Delmer A (2017) Relapsed chronic lymphocytic leukemia retreated with rituximab: interim results of the PERLE study. Leuk Lymphoma 58(6):1366–1375
Acknowledgments
The Population-based HAematological Registry for Observational Studies (PHAROS) is an initiative of the Haemato-Oncology Foundation for Adults in The Netherlands (HOVON), the institute of Medical Technology Assessment (iMTA) at the Erasmus University Rotterdam, and the Netherlands Comprehensive Cancer Organisation (IKNL). We are grateful to all participating centers, hematologists, research nurses, and data managers for their contributions and efforts that allowed for additional data collection.
Author information
Authors and Affiliations
Contributions
LvdS, AGD, and M-DL designed the study; LvdS analyzed the data; AGD provided statistical support; ECvdB collected the data; LvdS wrote the manuscript with contributions from all authors, who also interpreted the data, and read, commented on, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 221 kb)
Rights and permissions
About this article
Cite this article
van der Straten, L., Kater, A.P., Doorduijn, J.K. et al. Possible hampered effectiveness of second-line treatment with rituximab-containing chemotherapy without signs of rituximab resistance: a population-based study among patients with chronic lymphocytic leukemia. Ann Hematol 99, 1081–1091 (2020). https://doi.org/10.1007/s00277-020-03994-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-03994-8