Abstract
Purpose
To summarize the 5-year outcomes of drug-coated balloon (DCB) for the treatment of femoropopliteal lesions in patients with diabetes mellitus (DM) or chronic limb-threatening ischemia (CLTI) compared to non-DM and intermittent claudication (IC).
Methods
The IN.PACT Global study was a real-world prospective, multicenter, international, single-arm study that enrolled 1535 participants. Post hoc analyses were conducted for participants with DM (n = 560) versus non-DM (n = 842) and CLTI (n = 156) versus IC (n = 1246). Assessments included freedom from clinically driven target lesion revascularization (CD-TLR) through 60 months, a composite safety outcome (freedom from device- and procedure-related death through 30 days, and freedom from major target limb amputation and freedom from CD-target vessel revascularization within 60 months), and major adverse events (MAEs).
Results
Kaplan–Meier estimates of 60-month freedom from CD-TLR were 67.7% and 70.5% (p = 0.25) in the DM and non-DM cohorts; and 60.7% and 70.5% (p = 0.006) in the CLTI and IC cohorts. The Kaplan–Meier 60-month composite safety outcomes were 65.1% DM versus 68.9% non-DM (p = 0.12); 53.2% CLTI versus 69.1% IC (p < 0.001). Between DM and non-DM, MAE rates were not significantly different through 60 months except for all-cause mortality which was higher in DM (23.8% versus 16.6%; p < 0.001). Participants with CLTI had a higher cumulative incidence of major target limb amputation (6.8% versus 1.1%; p < 0.001) and all-cause mortality (37.4% versus 17.4%; p < 0.001) through 60 months compared to IC.
Conclusions
In this real-world study, 5-year reintervention rates following DCB angioplasty were similar between DM and non-DM, but mortality rates were expectedly higher in patients with DM. Reintervention, mortality, and amputation rates were all higher in CLTI patients compared to IC, which is consistent with the known frailty of this patient population.
Level of Evidence
Level 3, Non-randomized controlled cohort/follow-up study
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Revascularization plays a key role in the management of chronic limb-threatening ischemia (CLTI) patients, with or without diabetes mellitus (DM), and may also be indicated in those patients with DM suffering from lifestyle-limiting intermittent claudication (IC) and not responding to walking exercise training. The goal of revascularization is to save limbs and improve the quality of life [1, 2]. Endovascular interventions are currently recommended for all lesions less than 25 cm in length and may also be considered in patients deemed unfit for surgery [1]. Paclitaxel-coated devices have become increasingly popular for peripheral artery disease (PAD) management. In particular, paclitaxel drug-coated balloon (DCB) catheters are desirable candidates for the treatment of femoropopliteal arteries with the potential to reduce restenosis without leaving a stent behind. Multiple randomized controlled trials (RCTs) have demonstrated the safety and effectiveness of DCBs for the treatment of symptomatic femoropopliteal arterial disease compared to plain balloon angioplasty [3,4,5,6,7,8,9,10,11]. Single-arm prospective global studies further evaluated DCBs for real-world patients with longer, more complex lesions [12,13,14,15,16]. However, long-term DCB data on high-risk patient groups such as DM and CLTI are limited.
A post hoc analysis of the IN.PACT Global Study previously reported 1-year outcomes in real-world patients with CLTI treated with a paclitaxel DCB [17]. This present post hoc analysis evaluates 5-year outcomes following DCB angioplasty in IN.PACT Global Study participants with DM and CLTI compared to non-DM and IC, respectively.
Methods
Study Design
The real-world prospective, multicenter, international, single-arm IN.PACT Global study evaluated the safety and effectiveness of the IN.PACT Admiral DCB (Medtronic) for the treatment of atherosclerotic disease of the superficial femoral and/or popliteal artery. Sites and Principal Investigators are listed in Supplementary Table 1. Participants (N = 1535) were enrolled across 64 international sites from 2012 to 2014, of which 1406 participants were treated with the IN.PACT Admiral DCB and included in the clinical cohort that was used for the current analysis. Detailed study design and outcomes through 5 years have been reported previously [12,13,14, 18].
This post hoc analysis reports two cohorts 1) DM versus non-DM and 2) CLTI (Rutherford category [RC] 4 and 5) versus IC (RC 2 and 3). Of note, enrollment of patients with RC 5 (n = 36) was considered a protocol deviation in the study. Additionally, one RC1 participant was enrolled as a protocol deviation.
Participants were followed at discharge, 30 days, 6 months, 12 months and then annually through 60 months. Follow-up evaluations were conducted via clinical visits through 36 months and by phone at 48 and 60 months. To verify mortality information, investigational sites were asked to obtain vital status updates from participants who withdrew or were lost to follow-up. Vital status update results are labeled as such when included.
An independent Clinical Events Committee (CEC; Syntactx, New York, NY, USA) adjudicated all major adverse events (MAEs) including clinically driven target lesion revascularizations (CD-TLRs) and clinically driven target vessel revascularizations (CD-TVRs) through 60 months after the index procedure. The study was conducted in accordance with good clinical practice guidelines, the Declaration of Helsinki and all applicable country laws. The institutional review board or ethics committee at each participating site approved the study protocol. Informed consent was obtained from all participants prior to enrollment. The trial was registered on the National Institutes of Health website (ClinicalTrials.gov identifier: NCT01609296).
Outcome Measures
Freedom from CD-TLR was reported through 60 months. CD-TLR and CD-TVR were defined as any reintervention within the target lesion(s) or vessel(s), respectively, because of symptoms or drop of ankle-brachial index (ABI) of ≥20% or >0.15 when compared with post-index procedure baseline ABI. The composite safety outcome was defined as freedom from device- and procedure-related death through 30 days and freedom from major target limb amputation and CD-TVR within 60 months after the index procedure. Other assessments through 60 months included any TLR, any TVR, and the incidence of MAEs (all-cause mortality, CD-TVR, major target limb amputation, and target lesion thrombosis). Functional outcomes including primary and secondary sustained clinical improvement were reported through 36 months. Full definitions of outcome measures are described in the Supplementary Methods.
Statistics
All analyses were based on participants with evaluable data. Baseline demographics, clinical characteristics, and outcomes are reported or analyzed on a participant basis. Lesion and device characteristics are reported on a lesion and device basis, respectively. Data are summarized descriptively using percentages and frequencies for categorical variables and the mean, standard deviation (SD), and number of observations for continuous variables. Time-to-event outcomes are summarized with survival curves and survival probabilities using the Kaplan–Meier method with log-rank P values. Confidence intervals (95% CI) were derived for time-to-event outcomes using the log-log transformation. Outcomes are also described using the restricted mean survival time (RMST) with a time horizon of 1800 days and 95% CI without bias correction. A participant was considered part of the analysis set if the study DCB was introduced into the sheath, after the guidewire had successfully passed through the target lesion. Annual cutoffs used 360 days per year (e.g., 1800 days for the 5-year cut-off). Statistical significance was set at 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Population
DM Versus Non-DM
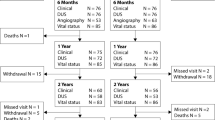
A participant flowchart is shown in Fig. 1. A total of 1402 participants with known DM status were stratified into the DM (n = 560) and non-DM (n = 842) cohorts. Overall, 60-month follow-up compliance was 96.4% and 97.4% for DM and non-DM, respectively. Participants in the DM cohort had higher burdens of obesity, hypertension, hyperlipidemia, coronary and carotid artery disease, renal insufficiency, concomitant below-the-knee disease, advanced PAD, and previous limb amputation (major or minor) as compared to the non-DM cohort (Table 1). The baseline lesion and procedural characteristics were similar between groups (Table 2), except for a higher calcification burden, including more severe calcification, in the DM (12.4%) compared to the non-DM cohort (8.7%). The mean lesion length was equivalent between DM and non-DM. Provisional stenting rates were 18.7% DM and 23.0% non-DM (p = 0.03).
IC Versus CLTI
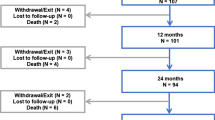
The flowchart for participants with CLTI (RC 4,5) and IC (RC 2,3) is shown in Fig. 2. Of the 1406 participants, 3 did not have known RC and 1 participant was in RC 1 at baseline. The remaining 1402 with known baseline RC and treated with the DCB were stratified into the CLTI (n = 156) and IC (n = 1246) cohorts. Overall follow-up compliance at 60 months was 94.6% in the CLTI cohort and 97.4% in the IC cohort. Participants in the CLTI cohort were significantly older, were more often women, had higher burdens of DM, renal insufficiency, concomitant below-the-knee vascular disease, previous limb amputation, and had lower ABI compared to the IC cohort (Table 3). There were also significant differences in the lesion characteristics (Table 4): compared to IC, CLTI participants had more popliteal involvement, higher calcification burden, smaller reference vessel diameter, and longer lesions (13.9±10.6 cm versus 11.9±9.4 cm; p = 0.01). Provisional stenting rates were similar between CLTI and IC.
Participant flowchart of IC and CLTI cohorts at baseline in the IN.PACT Global Study. Four participants in the clinical cohort were not eligible for this analysis: RC was not known for three participants and one participant was in RC 1 at baseline. CLTI, chronic limb-threatening ischemia; IC, intermittent claudication, RC, Rutherford category.
Follow-up Outcomes
DM Versus Non-DM
Freedom from CD-TLR through 60 months was 67.7% (95% CI: 63.2–71.8%) in DM participants compared to 70.5% (95% CI: 66.9–73.7%) in non-DM participants (p = 0.25) (Fig. 3A). The RMST to first CD-TLR was not significantly different between cohorts (Table 5). Primary and secondary sustained clinical improvement rates were available through 36 months and were significantly lower in the DM cohort compared to the non-DM cohort (Table 5). The 60-month Kaplan–Meier composite safety outcomes were not significantly different between DM and non-DM participants: 65.1% (95% CI: 60.5–69.3%) DM versus 68.9% (95% CI: 65.3–72.2%) non-DM; p = 0.12 (Table 5). Compared to the non-DM cohort, the DM cohort had a higher cumulative incidence of composite major adverse events through 60 months (49.8% [95% CI: 45.5–54.3%] versus 43.3% [95% CI: 39.8–46.9%]; p = 0.009) driven by a higher all-cause death rate (23.8% versus 16.6%; p < 0.001). The rates of the individual MAE components are shown in Table 5. The survival probability of all-cause mortality based on vital status update (after accounting for participants who withdrew or were lost to follow-up) was 75.3% (95% CI: 71.4–78.7%) in the DM cohort and 81.4% (95% CI: 78.5–83.9%) in the non-DM cohort (p = 0.004) (Fig. 3B).
(A) Kaplan–Meier estimate of freedom from clinically driven target lesion revascularization (CD-TLR) through 1800 days (60 months), and (B) Kaplan–Meier estimate of freedom from all-cause mortality through 1800 days (60 months) in the IN.PACT Global Study diabetic and non-diabetic cohorts treated with the IN.PACT Admiral DCB. Bars represent the 95% confidence intervals.
DM Subset Analysis
There was no significant difference in the 60-month cumulative incidence of CD-TLR (35.1% versus 30.4%; p = 0.53) or major target limb amputation (3.8% versus 1.6%; p = 0.16) between the insulin-dependent DM and non-insulin-dependent DM sub-cohorts. The cumulative incidence of all-cause mortality with vital status was higher in the insulin-dependent DM sub-cohort compared to non-insulin-dependent DM sub-cohort (30.9% versus 19.9%; p = 0.003) (Supplementary Fig. 1).
CLTI Versus IC
Freedom from CD-TLR through 60 months was significantly lower in the CLTI cohort (60.7%; 95% CI: 50.9–69.1%) compared to the IC cohort (70.5%; 95% CI: 67.6–73.2%; p = 0.006) (Fig. 4). The RMST to first CD-TLR was lower in CLTI versus IC (Table 6). Primary sustained clinical improvement through 36 months was lower in the CLTI cohort. However, no statistically significant difference was observed for secondary sustained clinical improvement between the two cohorts (Table 6). The composite safety outcome was significantly better in the IC cohort compared to CLTI (53.2% [95% CI: 43.5–62.0%] CLTI versus 69.1% [95% CI: 66.2–71.8%] IC; p < 0.001) (Table 6). The cumulative incidence of 60-month composite MAE was 65.4% (95% CI: 57.3–73.3%) CLTI versus 43.5% (94% CI: 40.6–46.4%) IC (p < 0.001) (Table 6). Rates of individual MAE components are shown in Table 6. Freedom from major target limb amputation was 93.2% (95% CI: 85.9–96.8%) and 98.9% (95% CI: 98.0–99.4%) in the CLTI and IC cohorts, respectively (p < 0.001) (Fig. 5A). The freedom from all-cause mortality with vital status update was 60.0% (95% CI: 51.7–67.4%) and 81.2% (95% CI: 78.9–83.3%) in the CLTI and IC cohorts, respectively (p < 0.001) (Fig. 5B).
Kaplan–Meier estimate of freedom from CD-TLR through 1800 days (60 months) in the IN.PACT Global Study IC and CLTI Cohorts treated with the IN.PACT Admiral DCB. Bars represent the 95% confidence intervals. CD-TLR, clinically driven target lesion revascularization; CLTI, chronic limb-threatening ischemia; IC, intermittent claudication.
(A) Kaplan–Meier estimate of freedom from major target limb amputation 1800 days (60 months) and (B) Kaplan–Meier estimate of freedom from all-cause mortality after vital status update through 1800 days (60 months) in the IN.PACT Global Study IC and CLTI cohorts treated with the IN.PACT Admiral DCB. Bars represent the 95% confidence intervals. CLTI, chronic limb-threatening ischemia; IC, intermittent claudication.
Participants with Both DM and CLTI
Freedom from CD-TLR through 60 months was 52.6% (95% CI: 38.7–64.8%) in participants with concomitant CLTI and DM (Fig. 6A). The RMST to the first CD-TLR was 1254.9±80.0 days. Through 60 months, the freedom from major target limb amputation was 90.7% (95% CI: 78.5–96.1%) and freedom from all-cause mortality with vital status update was 61.9% (95% CI: 50.3–71.5%) (Fig. 6B and C).
Subset analysis of participants with concomitant CLTI and DM in the IN.PACT Global Study. (A) Kaplan–Meier estimate of freedom from CD-TLR through 1800 days (60 months), (B) Kaplan–Meier estimate of freedom from major target limb amputation through 1800 days (60 months), and (C) Kaplan–Meier estimate of freedom from all-cause mortality after vital status update through 1800 Days (60 months). Bars represent the 95% confidence intervals. CLTI, chronic limb-threatening ischemia; DM, diabetes mellitus. CD-TLR, clinically driven target lesion revascularization.
Discussion
This post hoc analysis evaluated the long-term clinical effectiveness of a DCB in patients with DM and/or CLTI compared to patients without those conditions. The strengths of this study included the prospective enrollment, rigorous adjudication of adverse events and high rates of compliance follow-up. Reintervention and amputation rates were low through 5 years, but expectedly higher in patients with CLTI compared to IC. Primary sustained clinical improvement through 36 months was achieved in over 50% of patients with DM or CLTI, although it was lower compared to non-DM and IC participants. Overall long-term survival was lower in patients with DM and CLTI, compared to non-DM and IC, highlighting the frailty of these patients [19,20,21].
DM is a risk factor for PAD and accelerated PAD progression leading to more ischemic events [22, 23]. Similarly, the present study observed a higher percentage of CLTI among DM compared to non-DM at baseline. DM patients also had more comorbidities, including obesity, hypertension, hyperlipidemia, and renal insufficiency, and more extensive vascular disease including more severe calcification and concomitant below-the-knee disease. Nonetheless, DCB angioplasty demonstrated good 5-year clinical outcomes in patients with DM, with similar freedom from CD-TLR as non-DM. There is a paucity of real-world femoropopliteal studies that reported 5-year effectiveness and safety outcomes of DCB in DM patients. A few registries (BIOLUX P-III and Lutonix Global SFA) analyzed DCB outcomes in DM subsets; however, outcomes were reported only through 2 years [15, 24]. Long-term interaction effects between DM status and treatment modality (DCB versus plain balloon angioplasty) were examined in the IN.PACT SFA and EffPac RCTs, showing no statistically significant interaction effects for CD-TLR (IN.PACT SFA) or primary patency (EffPac) between DM status and treatment modality [4, 25].
In the present analysis, the 5-year cumulative incidence of major amputation remained low in both DM (2.5%) and non-DM (1.1%). These findings are notable considering that a significant number of amputations occur every year due to diabetes-related complications [26]. The current results are also favorable compared to other endovascular studies of DM patients. In a prospective registry of 765 patients (560 DM, 205 non-DM) undergoing endovascular therapy for symptomatic PAD, the above-the-ankle amputation rates were 5.6% in DM and 3.3% in non-DM patients [19]. Conversely, a retrospective study reported 5-year limb salvage rates of 84% DM and 93% non-DM overall, and 72% DM and 79% non-DM in patients presenting with CLTI after PTA/stent infrainguinal revascularization [27].
Five-year all-cause mortality was significantly higher in patients with DM (23.8%) compared to non-DM (16.6%) in the present study. Mueller et al. reported 5-year mortality rates of 10% non-DM and 23% DM in PAD patients who are < 75 years, and 38% non-DM and 52% DM in PAD patients who are ≥75 years [28]. These results were corroborated by a meta-analysis showing 5-year mortality rates ranging from 32 to 68% in DM patients versus 19 to 42% in non-DM patients (odds ratio 1.89, p < 0.001) with PAD [29]. In the present study, 44.5% of DM patients were insulin-dependent. The 5-year cumulative incidence of mortality with vital status update was significantly higher in the insulin-dependent sub-cohort compared to the non-insulin-dependent sub-cohort, and aligned with previous reports [30, 31]. A database analysis (N = 8022) reported a significantly increased risk of post-procedural mortality in insulin-dependent DM versus non-insulin-dependent DM patients (odds ratio 2.0, p = 0.009) [30].
In line with a prior report [32], CLTI participants had significantly higher baseline comorbidities than IC participants, as well as a higher incidence of long, calcified lesions. There was also more popliteal involvement in the CLTI compared to IC (41.8% versus 25.5%). This complexity was reflected in the significantly lower 5-year freedom from CD-TLR in CLTI (60.7%) compared to IC (70.5%). There are no published long-term TLR data after DCB angioplasty in CLTI patients. Therefore, the current comparisons are done with mixed populations consisting of both IC and CLTI. In a presentation, the 5-year freedom from CD-TLR was reported to be 68.5% and 70.3% in the DCB arms of the ILLUMENATE EU (mean lesion length 7.2 cm) and the ILLUMENATE Pivotal (mean lesion length 8.3 cm) RCTs [33]. However, those RCTs consisted of primarily IC patients with less complex lesions. Five-year freedom from CD-TLR was slightly higher in the AcoArt I RCT (77.5% in the DCB arm; mean lesion length 14.7 cm) [34] than the present study; however, AcoArt I DCB patients were younger, had less DM, and had fewer total occlusions (and calcification was not reported).
Despite the complexity, DCB angioplasty showed a sustained safety profile in the CLTI cohort. More than 50% of CLTI patients were free from the safety events through 5 years. In population-based studies, the long-term prognosis for CLTI patients is unfavorable, [35] with 5-year mortality rates higher than most cancers. A Medicare beneficiary study of 72,199 patients reported a 4-year mortality rate of 54% following CLTI diagnosis [36]. In a recent review article reporting on 4 to 5 years time horizons, mortality commonly exceeded 50%, but mortality was as high as 85% in patients >70 years undergoing amputation [35]. In the present study also, all-cause mortality was significantly higher in CLTI compared to IC (37.4% versus 17.4%). However, this rate is favorable compared to population-based studies, and is aligned with the 24.1–45.0% mortality rates reported for BEST-CLI and BASIL-2 trials at a median follow-up of 1.6-3.3 years after endovascular interventions [37, 38].
The 5-year major target limb amputation rates in the current study (6.8% CLTI, 1.1% IC) compare favorably to the 1.4%, 1.5%, and 2.3% rates in the DCB arms of the ILLUMENATE EU, ILLUMENATE Pivotal, and AcoArt I RCTs (33, 34), all of which enrolled primarily IC patients. At the time of writing this paper, no other global DCB studies have reported amputation rates through 5 years. In population-based studies, amputation rates are unacceptably high in CLTI patients, typically exceeding 15–20% at 1 year [35]. A prospective population-based study in the United Kingdom reported a 5-year amputation rate of 43.4% in CLTI patients, [20] while a pooled analysis from the Netherlands reported 5-year major amputation rates of 34.1% in CLTI patients with DM and 20.4% without DM [21]. Recently, the BEST-CLI trial reported above-ankle index-limb amputation rates of 14.2% to 14.9% at a median follow-up of 1.6 to 2.7 years after endovascular intervention [37]. However, a direct comparison between the present study and BEST-CLI is not possible due to differences in study design, endovascular modality (only 25–28% of BEST-CLI patients received a DCB), and patient demographics (more DM patients were included in BEST-CLI). Interestingly, in the present study, a subset analysis of CLTI patients with concurrent DM showed that 5-year freedom from major target limb amputation (90.7%) and freedom from all-cause mortality (61.9%) were not worse than the overall CLTI cohort, albeit with a lower rate of freedom from CD-TLR (52.6%), suggesting that while more reinterventions are required in this vulnerable subset, safety can be reasonably achieved.
An incremental increase in amputation rates with increasing RC has been well documented [35]. RC 6 was excluded in the present study, which may have contributed to the low major target limb amputation rate. Also, most patients were treated for RC 4. Nonetheless, the 6.8% 5-year major target limb amputation rate in CLTI patients (RC 4–5) with complex lesions is highly encouraging. Furthermore, there may be cost-benefit implications of DCB for CLTI patients. It has been shown that CLTI is associated with high healthcare costs [39]. A recent IN.PACT Global CLTI cost analysis reported that DCB treatment was associated with improved patient outcomes and significant cost savings in the Dutch and German healthcare systems [40]. The authors concluded that DCB is a cost-effective modality and likely the dominant treatment strategy for CLTI patients with femoropopliteal lesions.
Limitations
This was a non-blinded study with no comparator arm. The CLTI cohort was relatively small, partially enrolled as the result of protocol deviations, and no hypotheses were pre-specified to assess statistical power. This CLTI cohort comprised only patients with RC 4 and RC 5; RC 6 was excluded from the enrollment. In the overall study, imaging data were not available for all patients hence no anatomic outcomes were analyzed in these cohorts.
Conclusions
Results from this real-world study demonstrate encouraging 5-year reintervention and safety outcomes that are consistent with prior endovascular studies and the known increased risk profile of patients with DM and CLTI. DCB may be considered a treatment option for PAD patients with DM and/or CLTI; higher reintervention rates in patients with CLTI versus claudicants should be considered when determining follow-up plans.
References
Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the european society for vascular surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesendorsed by: the european stroke organization (ESO)the task force for the diagnosis and treatment of peripheral arterial diseases of the european society of cardiology (ESC) and of the european society for vascular surgery (ESVS). Eur Heart J. 2018;39(9):763–816.
Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69(6S):3S-125S.e40.
Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131(5):495–502.
Laird JA, Schneider PA, Jaff MR, Brodmann M, Zeller T, Metzger DC, et al. Long-term clinical effectiveness of a drug-coated balloon for the treatment of femoropopliteal lesions. Circ Cardiovasc Interv. 2019;12(6):e007702.
Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373(2):145–53.
Krishnan P, Faries P, Niazi K, Jain A, Sachar R, Bachinsky WB, et al. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136(12):1102–13.
Soga Y, Iida O, Urasawa K, Saito S, Jaff MR, Wang H, et al. Three-year results of the INPACT SFA Japan Trial comparing drug-coated balloons with percutaneous transluminal angioplasty. J Endovasc Ther. 2020;27(6):946–55.
Chen Z, Guo W, Jiang W, Wang F, Weiguo F, Zou Y, Deckers S, Li P, Popma JJ, Jaff MR. IN.PACT SFA clinical study using the IN.PACT admiral drug-coated balloon in a chinese patient population. J Endovasc Ther. 2019;26(4):471–8.
Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 2015;8:102–8.
Scheinert D, Schulte KL, Zeller T, Lammer J, Tepe G. Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve-month results from the BIOLUX P-I randomized trial. J Endovasc Ther. 2015;22(1):14–21.
Jia X, Zhang J, Zhuang B, Fu W, Wu D, Wang F, et al. Acotec drug-coated balloon catheter: randomized, multicenter, controlled clinical study in femoropopliteal arteries: evidence from the acoart I trial. JACC Cardiovasc Interv. 2016;9(18):1941–9.
Torsello G, Stavroulakis K, Brodmann M, Micari A, Tepe G, Veroux P, et al. Three-year sustained clinical efficacy of drug-coated balloon angioplasty in a real-world femoropopliteal cohort. J Endovasc Ther. 2020;27(5):693–705.
Micari A, Brodmann M, Keirse K, Peeters P, Tepe G, Frost M, et al. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain: 2-year results from the IN.PACT Global Study. JACC Cardiovasc Interv. 2018;11(10):945–53.
Zeller T, Brodmann M, Micari A, Keirse K, Peeters P, Tepe G, et al. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain. Circ Cardiovasc Interv. 2019;12(1):e007730.
Thieme M, Von Bilderling P, Paetzel C, Karnabatidis D, Perez Delgado J, Lichtenberg M, et al. The 24-month results of the Lutonix Global SFA Registry: worldwide experience with Lutonix drug-coated balloon. JACC Cardiovasc Interv. 2017;10(16):1682–90.
Schroe H, Holden AH, Goueffic Y, Jansen SJ, Peeters P, Keirse K, et al. Stellarex drug-coated balloon for treatment of femoropopliteal arterial disease-the ILLUMENATE global study: 12-month results from a prospective, multicenter, single-arm study. Catheter Cardiovasc Interv. 2018;91(3):497–504.
Reijnen M, van Wijck I, Zeller T, Micari A, Veroux P, Keirse K, et al. Outcomes after drug-coated balloon treatment of femoropopliteal lesions in patients with critical limb ischemia: a post hoc analysis from the IN.PACT Global Study. J Endovasc Ther. 2019;26(3):305–15.
Zeller T, Brodmann M, Ansel GM, Scheinert D, Choi D, Tepe G, et al. Paclitaxel-coated balloons for femoropopliteal peripheral arterial disease: final five-year results of the IN.PACT Global Study. EuroIntervention. 2022;18(11):e940–8.
Lee MS, Choi BG, Rha SW. Impact of diabetes mellitus on 5-year clinical outcomes following successful endovascular revascularization for peripheral artery disease. Vasc Med. 2020;25(1):33–40.
Howard DP, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell PM, et al. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015;132(19):1805–15.
Spreen MI, Gremmels H, Teraa M, Sprengers RW, Verhaar MC, Statius van Eps RG, et al. Diabetes is associated with decreased limb survival in patients with critical limb ischemia: pooled data from two randomized controlled trials. Diabetes Care. 2016;39(11):2058–64.
Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6(7):961–9.
Mwipatayi BP, Barry IP, Brodmann M, Zeller T, Varcoe RL, Moscovic M, et al. Twenty-four-month outcomes of drug-coated balloon in diabetic patients in the BIOLUX P-III registry: a subgroup analysis. Ann Vasc Surg. 2021;75:237–52.
Tepe G, Zeller T, Moscovic M, Corpataux JM, Christensen JK, Keirse K, et al. Paclitaxel-coated balloon angioplasty for the treatment of infrainguinal arteries: 24-month outcomes in the full cohort of BIOLUX P-III global registry. Cardiovasc Intervent Radiol. 2021;44(2):207–17.
Teichgraber U, Lehmann T, Ingwersen M, Aschenbach R, Zeller T, Brechtel K, et al. Long-term effectiveness and safety of femoropopliteal drug-coated balloon angioplasty: 5-year results of the randomized controlled EffPac trial. Cardiovasc Intervent Radiol. 2022;45(12):1774–83.
Mizelle RM Jr. Diabetes, race, and amputations. Lancet. 2021;397(10281):1256–7.
Abularrage CJ, Conrad MF, Hackney LA, Paruchuri V, Crawford RS, Kwolek CJ, et al. Long-term outcomes of diabetic patients undergoing endovascular infrainguinal interventions. J Vasc Surg. 2010;52(2):314-22.e1-4.
Mueller T, Hinterreiter F, Luft C, Poelz W, Haltmayer M, Dieplinger B. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg. 2014;59(5):1291–9.
Vrsalovic M, Vucur K, Vrsalovic Presecki A, Fabijanic D, Milosevic M. Impact of diabetes on mortality in peripheral artery disease: a meta-analysis. Clin Cardiol. 2017;40(5):287–91.
An TJ, Cochran RL, Di Capua J, Reid N, Walker TG. Insulin-dependent status influences post-procedural outcomes in diabetic patients following lower extremity endovascular intervention for peripheral arterial disease. Cardiovasc Intervent Radiol. 2021;44(8):1165–73.
Maca T, Mlekusch W, Doweik L, Budinsky AC, Bischof M, Minar E, et al. Influence and interaction of diabetes and lipoprotein (a) serum levels on mortality of patients with peripheral artery disease. Eur J Clin Invest. 2007;37(3):180–6.
Zettervall SL, Marshall AP, Fleser P, Guzman RJ. Association of arterial calcification with chronic limb ischemia in patients with peripheral artery disease. J Vasc Surg. 2018;67(2):507–13.
Brodmann M. 5-Year Safety and Efficacy Data from the ILLUMENATE EU and Pivotal RCTs. The Amputation Prevention Symposia (AMP). Chicago, August 11–14, 2021.
Xu Y, Liu J, Zhang J, Zhuang B, Jia X, Fu W, et al. Long-term safety and efficacy of angioplasty of femoropopliteal artery disease with drug-coated balloons from the AcoArt I trial. J Vasc Surg. 2021;74(3):756-62.e3.
Duff S, Mafilios MS, Bhounsule P, Hasegawa JT. The burden of critical limb ischemia: a review of recent literature. Vasc Health Risk Manag. 2019;15:187–208.
Mustapha JA, Katzen BT, Neville RF, Lookstein RA, Zeller T, Miller LE, et al. Disease burden and clinical outcomes following initial diagnosis of critical limb ischemia in the Medicare population. JACC Cardiovasc Interv. 2018;11(10):1011–2.
Farber A, Menard MT, Conte MS, Kaufman JA, Powell RJ, Choudhry NK, et al. Surgery or endovascular therapy for chronic limb-threatening Ischemia. N Engl J Med. 2022;387(25):2305–16.
Bradbury AW, Moakes CA, Popplewell M, Meecham L, Bate GR, Kelly L, et al. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. Lancet. 2023;401:17798.
Mustapha JA, Katzen BT, Neville RF, Lookstein RA, Zeller T, Miller LE, et al. Determinants of long-term outcomes and costs in the management of critical limb ischemia: a population-based cohort study. J Am Heart Assoc. 2018;7(16):e009724.
Pietzsch JB, Geisler BP, Iken AR, van Wijck IPS, Holewijn S, Reijnen M. Cost-effectiveness of urea excipient-based drug-coated balloons for chronic limb-threatening ischemia from femoropopliteal disease in the Netherlands and Germany. Cardiovasc Intervent Radiol. 2022;45(3):298–305.
Acknowledgement
The authors would like to acknowledge Sangeeta Yendrembam, PhD (Medtronic) for medical writing assistance; Stefanie Deckers, MS and Giulia Gatta, MS (Medtronic) for clinical support; and Kristin Hood, PhD (Medtronic) for technical review in accordance with Good Publication Practice guidelines (Battisti et al., Ann Intern Med. 2015;163:461-4).
Funding
Open access funding provided by Rijnstate. This study was funded by Medtronic. The funder of the study was involved in the study design, data collection, data analysis, data interpretation, and provided medical writing support. The authors had full access to all the data, interpretation, manuscript writing and had full and final responsibility for the decision to submit for publication. Authors received no specific funding for preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Michel M. P. J. Reijnen, MD, PhD, is a consultant for Bentley Innomed, Medtronic, Terumo Aortic, and W.L. Gore and Associates. Iris Van Wijck, MD, has nothing to disclose. Marianne Brodmann, MD, received speaking honoraria from Bard Peripheral Vascular, Biotronik, Medtronic, Spectranetics, and VIVA Physicians and is a consultant for Bard Peripheral Vascular, Biotronik, Medtronic, and Spectranetics. Antonio Micari, MD, PhD, is a compensated consultant for Medtronic and Boston Scientific Corp. Giovanni Torsello, MD, received grants and speaking honoraria from Biotronik, Boston Scientific Corp., Cordis, W.L. Gore & Associates and Medtronic. Seung-Woon Rha, MD, PhD, has nothing to disclose. Jeremiah Menk, MS, is a full-time employee of Medtronic. Thomas Zeller, MD, PhD, received speaking honoraria from Abbott Vascular, Bard Peripheral Vascular, Biotronik, Boston Scientific Corp, Cook Medical, Cordis, GLG, W.L. Gore & Associates, Medtronic, Philips, Spectranetics, Straub Medical, TriReme, Veryan, and VIVA Physicians; he is a consultant for Abbott Vascular, Bard Peripheral Vascular, Boston Scientific Corp, Cook Medical, W.L. Gore & Associates, Medtronic, and Spectranetics; and his clinic has received study funds or funds for research or clinical trials from 480 Biomedical, Abbott Vascular, B. Braun, Bard Peripheral Vascular, Bayer Pharma, Biotronik, Caveo Med, Contego Medical, Cook Medical, CSI, W.L. Gore & Associates, Innora, Intact Vascular, Medtronic, Mercator, Philips, Pluristem, Shockwave, Spectranetics, Terumo, TriReme, and Veryan.
Consent for Publication
For this type of study consent for publication is not required.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board or Ethics Committee at each study site approved the study protocol.
Information Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reijnen, M.M.P.J., van Wijck, I., Brodmann, M. et al. Five-Year Outcomes after Paclitaxel Drug-Coated Balloon Treatment of Femoropopliteal Lesions in Diabetic and Chronic Limb-Threatening Ischemia Cohorts: IN.PACT Global Study Post Hoc Analysis. Cardiovasc Intervent Radiol 46, 1329–1345 (2023). https://doi.org/10.1007/s00270-023-03478-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-023-03478-y