Abstract
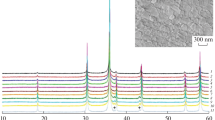
Using a conventional high-T furnace, the solid solutions between magnesiochromite and manganochromite, (Mg1−x Mn x )Cr2O4 with x = 0.00, 0.19, 0.44, 0.61, 0.77 and 1.00, were synthesized at 1,473 K for 48 h in open air. The ambient powder X-ray diffraction data suggest that the V–x relationship of the spinels does not show significant deviation from the Vegard’s law. In situ high-T powder X-ray diffraction measurements were taken up to 1,273 K at ambient pressure. For the investigated temperature range, the unit-cell parameters of the spinels increase smoothly with temperature increment, indicating no sign of cation redistribution between the tetrahedral and octahedral sites. The V–T data were fitted with a polynomial expression for the volumetric thermal expansion coefficient (\( \alpha_{T} = a_{0} + a_{1} T + a_{2} T^{ - 2} \)), which yielded insignificant a 2 values. The effect of the composition on a 0 is adequately described by the equation a 0 = [17.7(8) − 2.4(1) × x] 10−6 K−1, whereas that on a 1 by the equation a 1 = [8.6(9) + 2.1(11) × x] 10−9 K−2.






Similar content being viewed by others
References
Biernacki L, Pokrzywnicki S (1999) The thermal decomposition of manganese carbonate. J Thermal Anal Calorim 55:227–232
Binns RA, Davis RJ, Reed SBJ (1969) Ringwoodite natural (Mg, Fe)2SiO4 spinel in Tenham meteorite. Nature 221:943–944
Catti M, Freyria Fava F, Zicovich C, Dovesi R (1999) High-pressure decomposition of MCr2O4 spinels (M = Mg, Mn, Zn) by ab initio methods. Phys Chem Miner 26:389–395
Della Giusta A, Ottonello G (1993) Energy and long-range disorder in simple spinels. Phys Chem Miner 20:228–241
Dick HJB, Bullen T (1984) Chromian spinel as a petrogenetic indicator in abyssal and alpine-type peridotites and spatially associated lavas. Contrib Miner Petrol 86:54–76
Fei Y (1995) Thermal expansion. In: Ahrens TJ (eds) Mineral physics and crystallography: a handbook of physical constants. American Geophysical Union, Washington, DC, Shelf 2, pp 29–44
Fiorani D, Viticoli S (1980) Investigations on magnetically dilute Co x Zn1−x Rh2O4 spinel solid solutions. J Phys Chem Solids 41:1041–1045
Freyria Fava F, Baraille I, Lichanot A, Larrieu C, Dovesi R (1997) On the structural, electronic and magnetic properties of MnCr2O4 spinel. J Phys: Condens Matter 9:10715–10724
Gilewicz-Wolter J, Zurek Z, Dudala J, Lis J, Homa M, Wolter M (2005) Diffusion of chromium, manganese, and iron in MnCr2O4 spinel. J Phase Equilib Diff 26:561–564
Grimes NW, Al-Ajaj EA (1992) Low-temperature thermal expansion of spinel. J Phys: Condens Matter 4:6375–6380
Gross J, Treiman AH (2011) Unique spinel-rich lithology in lunar meteorite ALHA81005: origin and possible connection to M3 observations of the farside highlands. J Geophys Res 116:E10009
Harrison RJ, Redfern SAT, HStC O’Neill (1998) The temperature dependence of the cation distribution in synthetic hercynite (FeAl2O4) from in situ neutron structure refinements. Am Miner 83:1092–1099
Hastings JM, Corliss LM (1962) Magnetic structure of manganese chromite. Phys Rev 126:556–565
Hazen RM, Yang H (1999) Effect of cation substitution and order-disorder on P-V-T equations of state of cubic spinels. Am Miner 84:1956–1960
He Q, Liu X, Hu X, Li S, Wang H (2011) Solid solution between lead fluorapatite and lead fluorvanadate apatite: mixing behavior, Raman feature and thermal expansivity. Phys Chem Miner 38:741–752
Hill RJ, Craig JR, Gibbs GV (1979) Systematics of the spinel structure type. Phys Chem Miner 4:317–339
Hu X, Liu X, He Q, Wang H, Qin S, Ren L, Wu C, Chang L (2011) Thermal expansion of andalusite and sillimanite at ambient pressure: a powder X-ray diffraction study up to 1,000°C. Miner Mag 75:363–374
Inoue T, Tanimoto Y, Irifune T, Suzuki T, Fukui H, Ohtaka O (2004) Thermal expansion of wadsleyite, ringwoodite, hydrous wadsleyite and hydrous ringwoodite. Phys Earth Planet Inter 143–144:279–290
Jacob KT, Fitzner K (1977) Ion-exchange equilibria between (Mn, Co)O solid solution and (Mn, Co)Cr2O4 and (Mn, Co)Al2O4 spinel solid solutions at 1100°C. J Mater Sci 12:481–488
Jacob KT, Patil R (1998) Activities in the spinel solid solution Fe x Mg1−x Al2O4. Metall Mater Trans B29:1241–1248
Jung IH (2006) Critical evaluation and thermodynamic modeling of the Mn–Cr–O system for the oxidation of SOFC interconnect. Solid State Ionics 177:765–777
Klemme S, Ahrens M (2007) Low-temperature heat capacities of MgAl2O4 and spinels of the MgCr2O4-MgAl2O4 solid solution. Phys Chem Miner 34:59–72
Kovtunenko PV (1997) Defect formation in spinels in oxygen nonstoichiometry (a review). Glass Ceram 54:143–148
Lenaz D, Skogby H, Princivalle F, Hålenius U (2004) Structural changes and valence states in the MgCr2O4-FeCr2O4 solid solution series. Phys Chem Miner 31:633–642
Lenaz D, Skogby H, Princivalle F, Hålenius U (2006) The MgCr2O4-MgFe2O4 solid solution series: effects of octahedrally coordinated Fe3+ on T-O bond lengths. Phys Chem Miner 33:465–474
Levy D, Artioli G (1998) Thermal expansion of chromites and zinc spinels. Mater Sci Forum 278–281:390–395
Levy D, Diella V, Dapiaggi M, Sani A, Gemmi M, Pavese A (2004) Equation of state, structural behavior and phase diagram of synthetic MgFe2O4, as a function of pressure and temperature. Phys Chem Miner 31:122–129
Levy D, Diella V, Pavese A, Dapiaggi M, Sani A (2005) P-V equation of state, thermal expansion, and P-T stability of synthetic zincochromite (ZnCr2O4 spinel). Am Miner 90:1157–1162
Liu X, Prewitt CT (1990) High-temperature x-ray diffraction study of Co3O4: transition from normal to disordered spinel. Phys Chem Miner 17:168–172
Liu L, Zhang J, Green HW II, Jin Z, Bozhilov KN (2007) Evidence of former stishovite in metamorphosed sediments, implying subduction to > 350 km. Earth Planet Sci Lett 263:180–191
Liu X, Wang S, He Q, Chen J, Wang H, Li S, Peng F, Zhang L, Fei Y (2012) Thermal elastic behavior of CaSiO3-walstromite: a powder X-ray diffraction study up to 900°C. Am Miner (in press). doi:10.2138/am.2012.3689
Moriwake H, Tanaka I, Oba F, Koyama Y, Adachi H (2002) Formation energy of Cr/Al vacancies in spinel MgCr2O4 and MgAl2O4 by first-principles calculations. Phys Rev B65:153103
O’Neill HStC, Dollase WA (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4 and the temperature dependence of the cation distribution in ZnAl2O4. Phys Chem Miner 20:541–555
O’Neill HStC, Navrotsky A (1983) Simple spinels: crystallographic parameters, cation radii, lattice energies, and cation distribution. Am Miner 68:181–194
O’Neill HStC, Navrotsky A (1984) Cation distribution and thermodynamic properties of binary spinel solid solutions. Am Miner 69:733–753
O’Neill HStC, Redfern SAT, Kesson S, Short S (2003) An in situ neutron diffraction study of cation disordering in synthetic qandilite Mg2TiO4 at high temperatures. Am Miner 88:860–865
Paraskevopoulos GM, Economou M (1981) Zoned Mn-rich chromite from podiform type chromite ore in serpentinites of northern Greece. Am Miner 66:1013–1019
Park JH, Jung I-H, Lee S-B (2009) Phase diagram study for the CaO-SiO2-Cr2O3–5 mass. % MgO-10mass. % MnO system. Met Mater Int 15:677–681
Pieters CM, Besse S, Boardman J, Buratti B, Cheek L, Clark RN, Combe JP, Dhingra D, Goswami JN, Green RO, Head JW, Isaacson P, Klima R, Kramer G, Lundeen S, Malaret E, McCord T, Mustard J, Nettles J, Petro N, Runyon C, Staid M, Sunshine J, Taylor LA, Thaisen K, Tompkins S, Whitten J (2011) Mg-spinel lithology: a new rock-type on the lunar farside. J Geophys Res 116:E00G08
Qu W, Jian L, Hill JM, Ivey DG (2006) Electrical and microstructural characterization of spinel phases as potential coatings for SOFC metallic interconnects. J Power Sources 153:114–124
Raccah PM, Bouchard RJ, Wold A (1966) Crystallographic study of chromium spinels. J Appl Phys 37:1436–1437
Rosén E, Muan A (1966) Stability of MgAl2O4 at 1400°C as derived from equilibrium measurements in CoAl2O4-MgAl2O4 solid solutions. J Am Ceram Soc 49:107–108
Ross NL, Navrotsky (1987) The Mg2GeO4 olivine-spinel phase transition. Phys Chem Miner 14:473–481
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767
Skinner BJ (1966) Thermal expansion. In: Clark SP (eds) Handbook of physical constants. Geol Soc Am Mem, pp 75–95
Smyth JR, Jacobsen SD, Hazen RM (2000) Comparative crystal chemistry of orthosilicate minerals. In: Hazen RM, Downs RT (eds) High-temperature and high-pressure crystal chemistry. Reviews in mineralogy and geochemistry, vol 41. Mineralogical Society of American, Washington, DC, pp 187–209
Song SH, Yuan ZX, Xiao P (2003) Electrical properties of MnCr2O4 spinel. J Mater Sci Lett 22:755–757
Stefan E, Irvine JTS (2011) Synthesis and characterization of chromium spinels as potential electrode support materials for intermediate temperature solid oxide fuel cells. J Mater Sci 46:7191–7197
Suzuki I, Kumazawa M (1980) Anomalous thermal expansion in spinel MgAl2O4. Phys Chem Miner 5:279–284
Yamanaka T (1986) Crystal structures of Ni2SiO4 and Fe2SiO4 as a function of temperature and heating duration. Phys Chem Miner 13:227–232
Yamanaka T, Takéuchi Y (1983) Order-disorder transition in MgAl2O4 spinel at high temperatures up to 1700°C. Z Kristallogr 165:65–78
Acknowledgments
We thank Professor J. Chen for her assistance with the TEM characterization. We are grateful to two anonymous reviewers and Professor M. Matsui who provided us with very constructive comments that significantly improved our paper. This investigation was financially supported by the Fundamental Research Funds for the Central Universities from the Administer of Education of P. R. China (to X. Liu) and the National Natural Science Foundation of China (Grant #41090371).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Liu, X., Fei, Y. et al. In situ high-temperature powder X-ray diffraction study on the spinel solid solutions (Mg1−x Mn x )Cr2O4 . Phys Chem Minerals 39, 189–198 (2012). https://doi.org/10.1007/s00269-011-0474-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-011-0474-8